General Pediatrics
Session: General Pediatrics 4
361 - Group well child care for mothers in treatment for opioid use disorder: Implementation outcomes
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 361
Publication Number: 361.1666
Publication Number: 361.1666

Neera Goyal, MD MSc (she/her/hers)
Professor of Pediatrics
Nemours Children's Hospital
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: For mothers in treatment for opioid use disorder (OUD), group well child care (WCC) may enhance pediatric care effectiveness and engagement
Objective: Describe implementation of group WCC integrated into maternal OUD treatment
Design/Methods: A 2-arm cluster-randomized controlled trial at a single, urban, university-affiliated maternal OUD treatment program will evaluate the effectiveness and implementation of co-located group WCC. Randomization is clustered on child date of birth, with a 1:1 ratio assigned to intervention vs. control (routine WCC from an academic, pediatric practice). Study recruitment began in December 2021 and is ongoing. Eligible participants are: ≥ 18 years old, pregnant ≥ 28 weeks or < 12 months postpartum, English-speaking, and in treatment for OUD. Exclusion criteria include preterm delivery < 32 weeks, major congenital anomalies , and severe maternal psychological impairment. The intervention is co-located group WCC, defined as the provision of WCC on-site at the maternal OUD treatment program with a group of similarly aged children using a longer visit duration and group discussion. Routine immunizations, screenings, and anticipatory guidance are included in the group WCC visit, as well as specific emphasis on maternal wellness and recovery. Each group WCC visit is scheduled for 2 hours and led by one of 2 pediatricians and a nurse practitioner with OUD treatment expertise. Implementation of the intervention has been led by a multidisciplinary committee comprised of pediatric and OUD treatment staff. Preliminary intervention feasibility, acceptability, and fidelity were assessed using descriptive statistics, including means and percent frequencies Intervention participants also completed brief, semi-structured interviews about their experience.
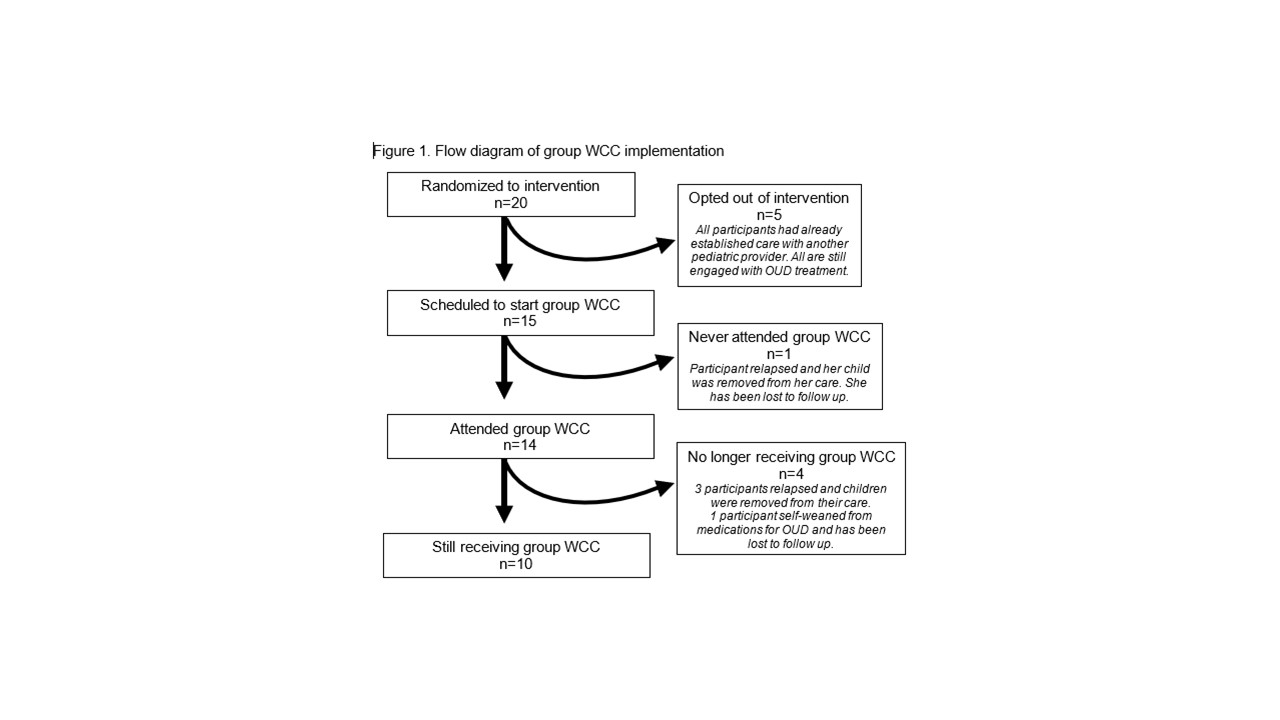
Results: Of 38 enrolled mothers, 20 have been randomized to group WCC (Table 1). Within this study arm, 5 opted out of the intervention after randomization and 15 agreed to receive group WCC (75% acceptance rate, see Figure 1). Of 15 participants scheduled for group WCC, 93% attended ≥ 1 group WCC visit, with a mean of 2.8 group WCC visits per participant. Intervention fidelity has been high, with a median of 2 participants per group WCC visit (IQR 2, 3), and a mean visit duration of 114 minutes. To date, exploratory interviews suggest high satisfaction with care and support intervention acceptability (Table 2).
Conclusion(s): Group WCC co-located with maternal OUD treatment is acceptable and may be associated with increased satisfaction with pediatric care. However, maternal relapse presents a significant challenge to implementation.
.jpg)
.jpg)
