Neonatology
Session: Neonatal Infectious Diseases/Immunology 1
591 - Loss of myeloid-KLF2 mediates LPS-induced mortality in neonatal pups by NLRP3 inflammasome signaling and altered neutrophil aging
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 591
Publication Number: 591.100
Publication Number: 591.100

Devashis Mukherjee, MD, MS (he/him/his)
Assistant Professor
Case Western Reserve University School of Medicine
Cleveland, Ohio, United States
Presenting Author(s)
Background: Preterm neonates have high mortality from sepsis. There is a knowledge gap of how preterm birth disrupts activation and migration of myeloid cells like neutrophils. KLF2 inhibits myeloid cell activation by repressing NF-kB. NF-kB controls NLRP3 inflammasome transcription. In a murine model of myeloid-KLF2 deletion (MKO), we have shown that postnatal day (P) 4 MKO pups have higher lipopolysaccharide (LPS)-induced mortality than controls, whereas there is 100% survival in both groups after LPS at P12. P4 and P12 reflect preterm and term physiology, respectively.
Objective: To determine role of neutrophil-NLRP3 and neutrophil migration in LPS-induced mortality in P4 MKO pups.
Design/Methods: Serum from facial vein blood was used for IL-1β ELISA. Neutrophils were obtained by immunomagnetic selection of bone marrow (BM) cells and treated with LPS and nigericin to prime and activate NLRP3 inflammasome. NLRP3 protein expression was measured via Western blotting. Neutrophil-IL-1β release was measured by ELISA in the supernatant. In vivo NLRP3 inhibition was done by daily MCC950 injections from P1-P4. BM cells were sorted by flow cytometry for the CD45+/Ly6G+ (neutrophil) fraction and analyzed for CXCR2, CXCR4, and CD62L surface expression.
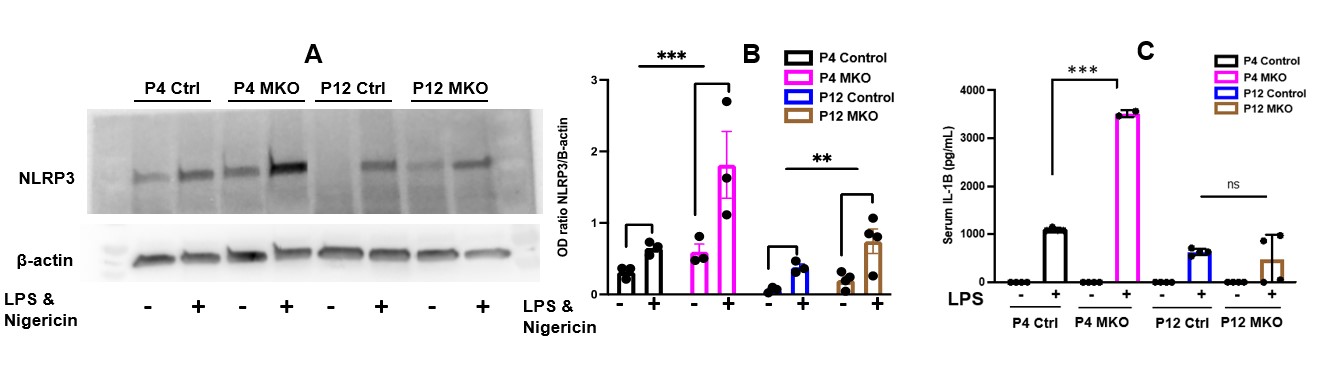
Results: MKO pups have significantly increased serum IL-1β, neutrophil-specific NLRP3 priming (protein expression), and activation (IL-1β release) in response to LPS than controls, and this is more pronounced at P4>P12 (Fig. 1). In vivo NLRP3 inhibition with MCC950 led to 100% survival after LPS in P4 MKO pups. At P4, the BM neutrophil fraction is lower, and the circulating neutrophil fraction is higher in MKO pups than in controls. BM neutrophils also decrease faster in response to LPS in P4 MKO vs control pups. At P12, there are no differences in BM neutrophil fraction between MKO and control pups at any time point (Fig. 2). CXCR4, which is essential for retaining BM neutrophils and homing aged neutrophils to BM, is lower at P4 vs P12 and only at P4, lower in MKO pups vs. controls (Fig. 3). At P4, MKO neutrophils have lower surface expression of CD62L vs controls; CD62L expression decreases with neutrophil aging.
Conclusion(s): Loss of myeloid-KLF2 leads to increased LPS-induced mortality only at an earlier postnatal age by NLRP3 priming and activation, and NLRP3 inhibition rescues survival. Loss of myeloid-KLF2 leads to accelerated neutrophil aging in P4 pups and persistence of these aged, inflammatory neutrophils by antagonizing CXCR4 expression and impairing their return to the BM. Further studies will focus on interaction of KLF2 with NLRP3 and CXCR4 at P4 and P12.
.jpg)
.jpg)
