Nephrology
Session: Nephrology 4
26 - Preliminary Findings From the Phase 2 EPPIK Study of Sparsentan in Pediatric Patients With Selected Proteinuric Glomerular Diseases
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 26
Publication Number: 26.1772
Publication Number: 26.1772

Crystal Traylor, MSN, APRN (she/her/hers)
Senior Medical Science Liaison
Travere Therapeutics, Inc.
Baton Rouge, Louisiana, United States
Presenting Author(s)
Background: Sparsentan (SPAR) is a novel, nonimmunosuppressive, single-molecule, dual endothelin angiotensin receptor antagonist (DEARA) approved by the US Food and Drug Administration for the treatment of adults with immunoglobulin A (IgA) nephropathy (IgAN) at risk of rapid disease progression and is being investigated for focal segmental glomerulosclerosis (FSGS).
Objective: The ongoing phase 2 EPPIK study is examining the safety and long-term antiproteinuric and nephroprotective potential of SPAR in pediatric patients with FSGS, minimal change disease (MCD), IgAN, IgA vasculitis nephritis (IgAVN), and Alport syndrome (AS). Here we report preliminary 12-week findings.
Design/Methods: This open-label, single-arm, multicenter trial is evaluating the safety, efficacy, and pharmacokinetics of SPAR in ≈30 patients aged 1 to < 18 years with FSGS and/or MCD (population 1) and ≈27 patients aged 2 to < 18 years with IgAN, IgAVN, or AS (population 2) over 108 weeks, with a 4-week safety follow-up. SPAR is administered once daily in a liquid formulation with dose adjusted to body weight. Patients receiving renin-angiotensin-aldosterone system inhibitors undergo a 2-week washout prior to study medication start (baseline). Primary endpoints include safety and efficacy (change in urine protein-to-creatinine ratio [UPCR] from baseline over 108 weeks).
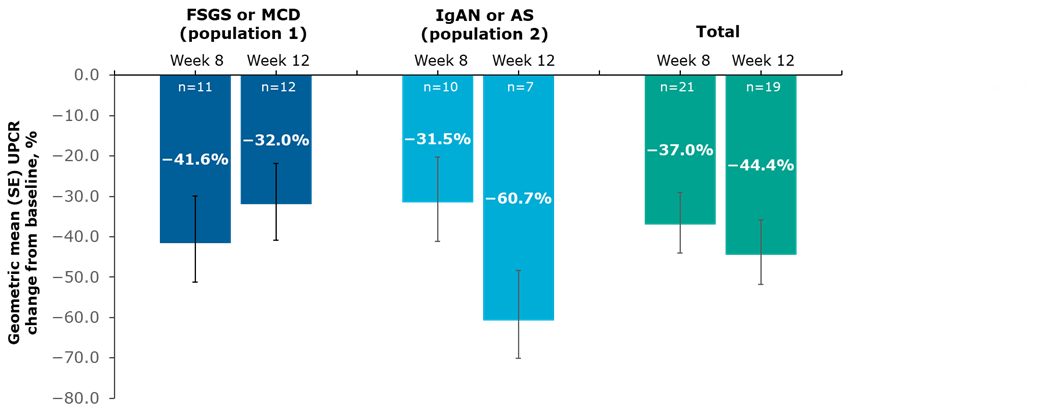
Results: At data cutoff (April 5, 2023), 23 participants had received ≥1 dose of SPAR. Baseline characteristics are shown in the Table. For these analyses, population 2 comprised patients with IgAN or AS, as no one with IgAVN was recruited. Geometric mean UPCR decreases from baseline at 12 weeks were −32.0% and −60.7% in populations 1 and 2, respectively, and −44.4% overall (Figure). SPAR treatment was safe and generally well tolerated.
Conclusion(s): SPAR treatment reduced proteinuria over the initial 12 weeks in pediatric patients with a range of proteinuric glomerular diseases. SPAR was safe and generally well tolerated, consistent with findings from ongoing FSGS and adult IgAN trials.
.png)