Neonatology
Session: Neonatal Follow-up 3
539 - Medication Profile Associated with 2- year Neurodevelopmental Outcomes in Infants with Moderate or Severe Hypoxic Ischemic Encephalopathy (HIE): A secondary analysis of the HEAL trial.
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 539
Publication Number: 539.3210
Publication Number: 539.3210

Sarah E. Kolnik, MD MBA (she/her/hers)
Assitant Professor of Pediatrics
University of Washington - Seattle Children's Hospital
Seattle, Washington, United States
Presenting Author(s)
Background: Little data exist on the post-discharge utilization of medications for neonates with HIE after therapeutic hypothermia (TH), the trajectory of medication use over time, and whether in-hospital markers of severity of injury correlate with medication use after discharge.
Objective: To determine if the neurological exam after rewarming provides information on the needs of post-discharge medication use and to characterize medication exposures over the first two years in neonates with moderate of severe HIE stratified by ND impairment (NDI) at 22-36 months of age.
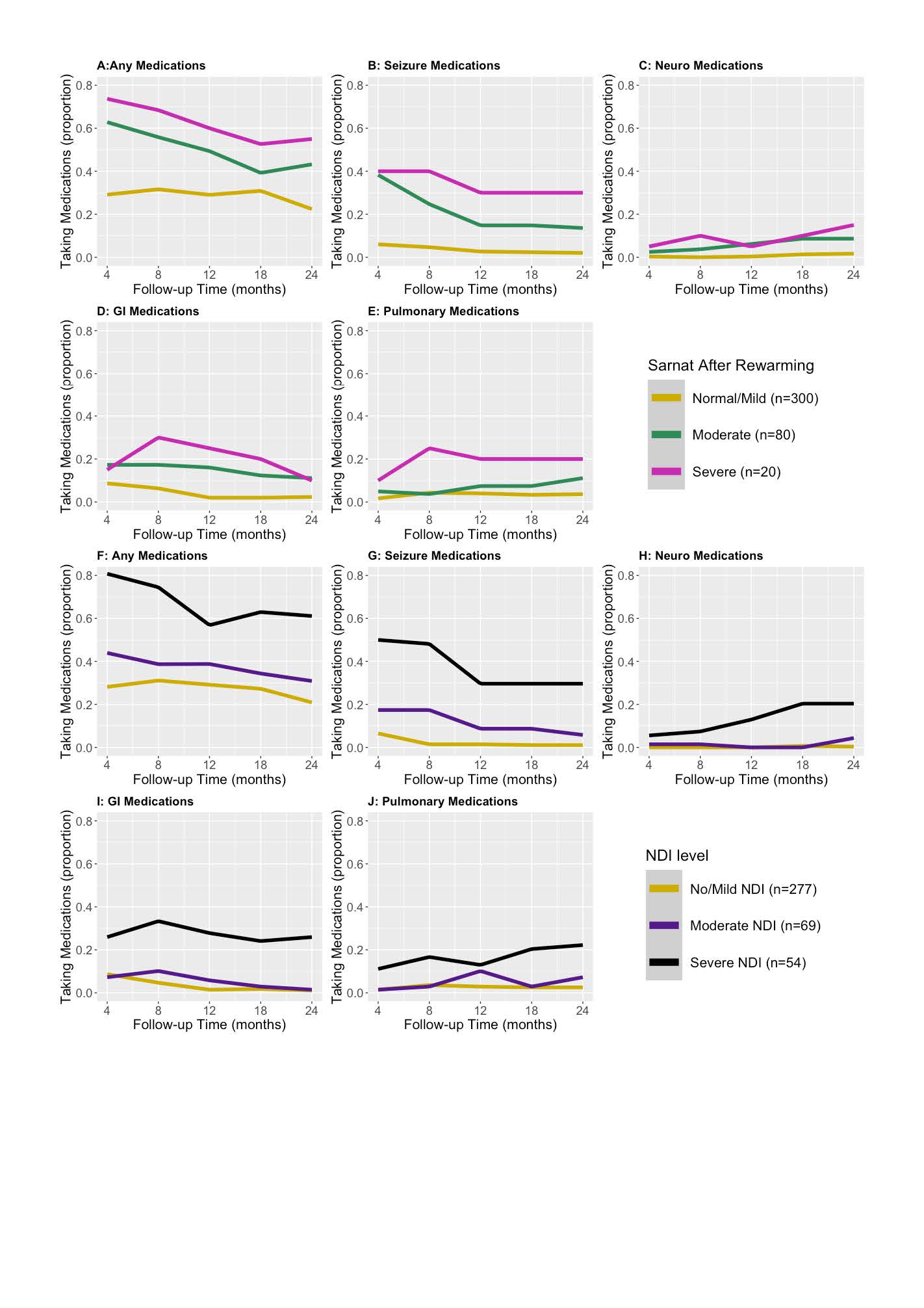
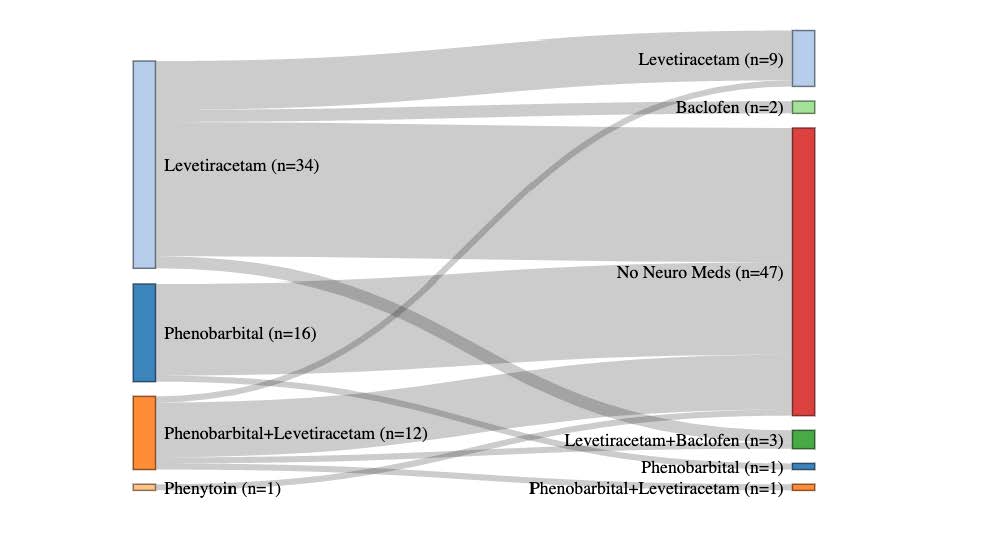
Design/Methods: A secondary analysis of data from infants with moderate or severe HIE enrolled in the High-dose Erythropoietin for Asphyxia and encephaLopathy (HEAL) Trial that tested erythropoietin plus therapeutic hypothermia for neuroprotection. Parents were asked about their child’s medication usage at 4, 8, 12, 18, and 24 months of age. The primary outcome was medication exposures in the first 2 years after birth. Medication utilization was stratified both prospectively by severity of encephalopathy after rewarming and retrospectively by Bayley Scales of Infant Development (BSID III) scores at 22-36 months of age. Neurological medication usage at 24-months for infants discharged on anti-seizure medications (ASMs) was displayed using a Sankey plot.
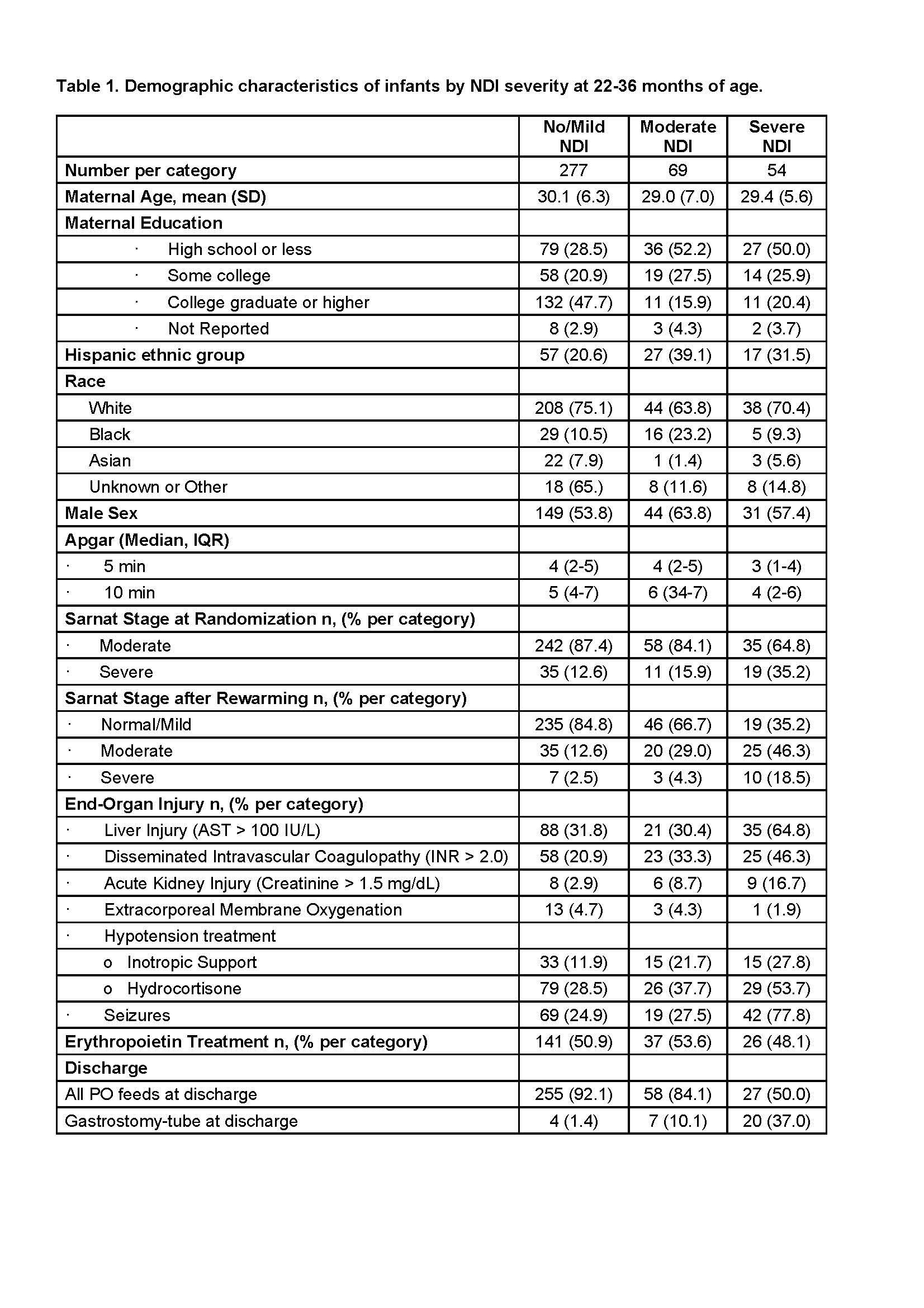
Results: Of 501 enrolled patients, 436 (87%) survived, of whom responses regarding medication utilization were available for 400 (92%) on at least one occasion. Baseline demographics of the cohort were described by NDI severity as determined by BSID III scores (Table 1). Trajectories of medication usage over time, stratified by severity of neurological exam on day 5 and NDI at 22-36 months are displayed in Fig 1A-E and 1F-J, respectively. Medication exposure over time correlated with worse day 5 neurological exam and NDI severity (Fig 1). ASM use decreased after discharge in severely and moderately affected participants while other neurology medications increased (Fig 1). Most patients discharged on ASMs were transitioned off by 22-36 months (Fig 2).
Conclusion(s): Medication utilization in infants with moderate or severe HIE after discharge is highest in those with abnormal exam after rewarming and those with severe NDI at 22-36 months of age. While the use of ASMs after discharge decreased by 2 years of age, the use of other neurological and pulmonary medication increased in those with severe NDI. Further investigation into the factors contributing to medication use after discharge in this high-risk population is needed.