Neonatology
Session: Neonatal Infectious Diseases/Immunology 3
629 - The efficacy of antibiotic regimens for the treatment of sepsis among young infants aged <60 days: a systematic review and meta-analysis.
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 629
Publication Number: 629.217
Publication Number: 629.217

Krysten North, MD, MPH
Instructor
Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background: Sepsis is a leading cause of death among young infants, accounting for about 420,00 neonatal deaths annually. Timely treatment with an appropriate antibiotic regimen is critical to prevent disease progression, sequelae, and mortality. Antibiotic stewardship is critical in this age of antimicrobial resistance.
Objective: To conduct a systematic review of randomized controlled trials among infants aged < 60 days comparing antibiotic regimens to treat sepsis or possible serious bacterial infection (PSBI).
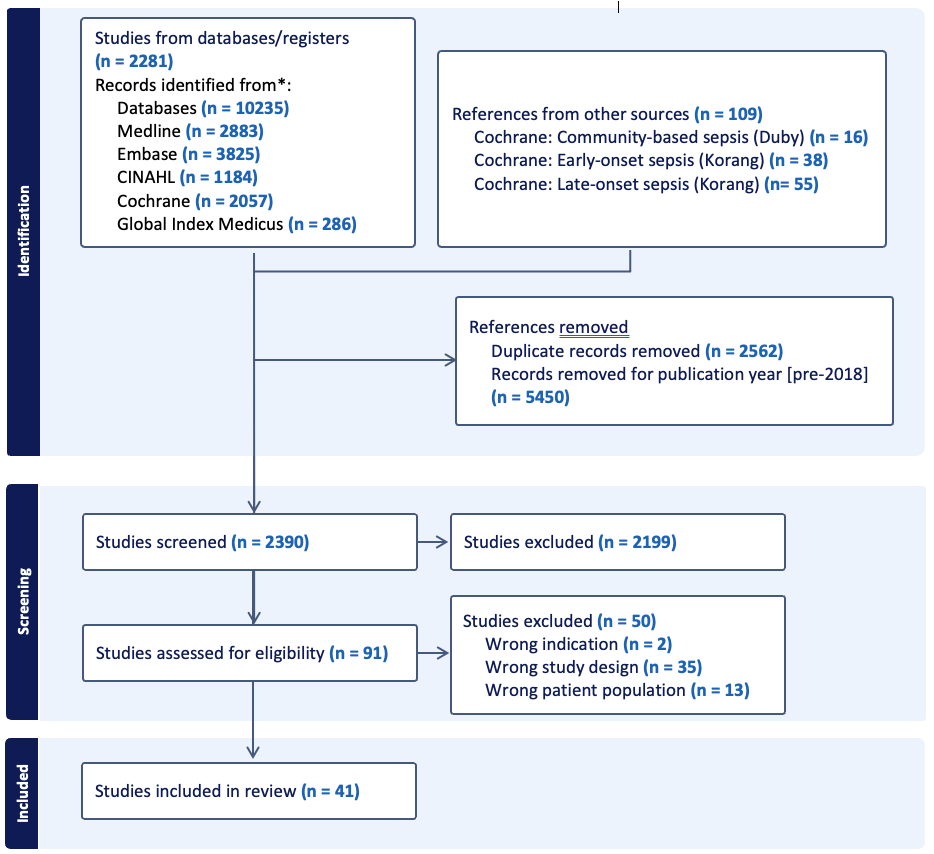
Design/Methods: We conducted a systematic review using standard Cochrane methods. We searched MEDLINE, Embase, CINAHL, the WHO Global Index Medicus, and the Cochrane Central Registry of Trials in April 2023 for randomized controlled trials (RCTs) of antibiotic efficacy for sepsis treatment in infants < 60 days. We updated previous Cochrane reviews of antibiotics for neonatal sepsis (Duby 2019, Korang 2021a and b), and conducted searches from 2018 onwards. Sepsis or PSBI definitions were classified per trialists. We conducted study screening, data extraction, and risk of bias assessment in duplicate. We grouped studies based upon similarity of the comparator arm to current WHO-recommended antibiotic regimens. We conducted random effects meta-analysis using RevMan and determined certainty of evidence using GRADE.
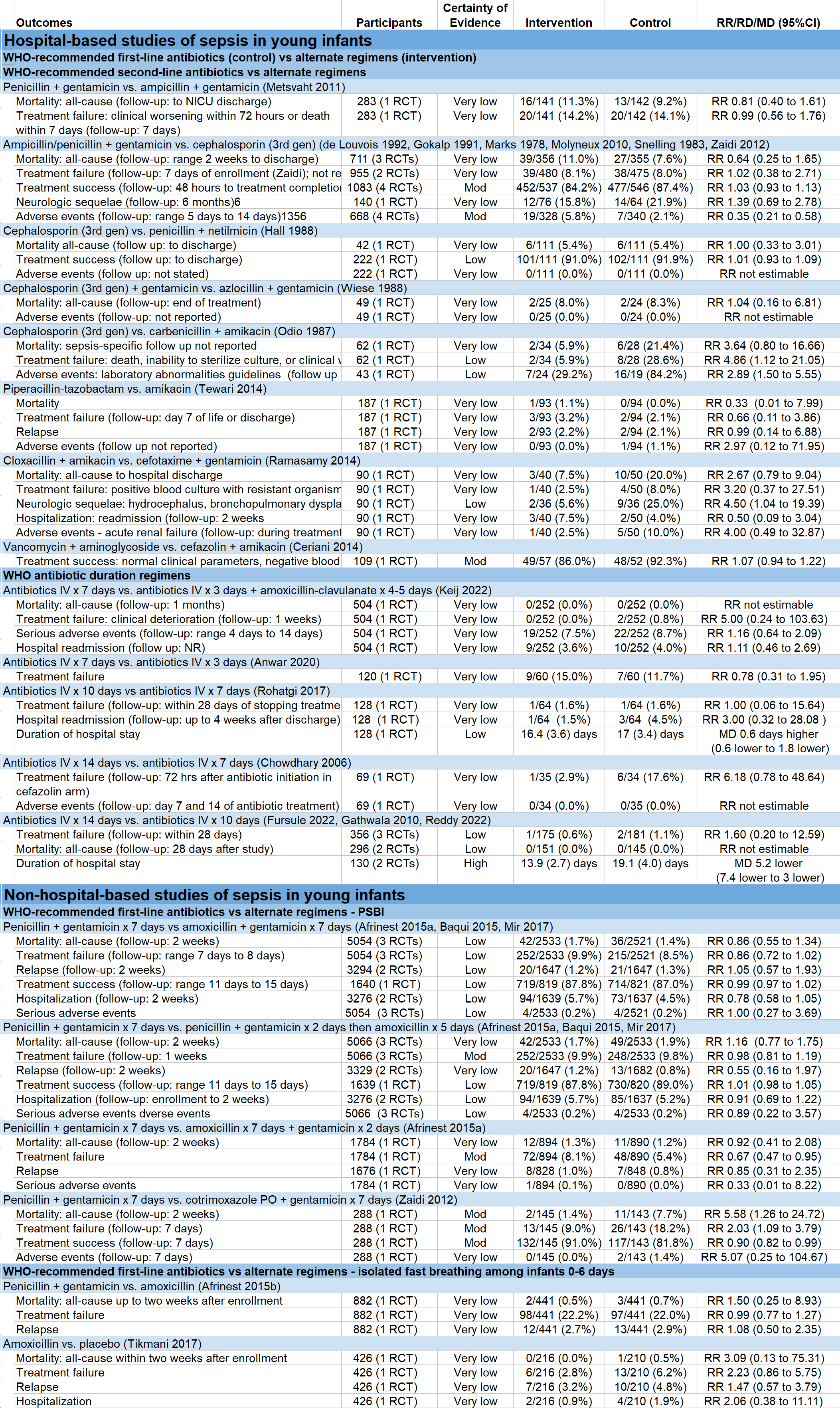
Results: Of 2390 screened studies, we included 41 RCTs of 181,964 infants in 42 countries from 1978 to 2022 study years (Table 1). Thirty trials were hospital-based and 11 were not based in hospitals. Of the hospital-based studies, 13 compared WHO first-line regimens of ampicillin/penicillin + gentamicin versus alternatives. Of the community-based studies, 5 compared penicillin and gentamicin to simplified oral regimens. Study comparison outcomes and certainty of evidence are shown in table 2. Most comparisons of antibiotic regimens were deemed low or very low certainty of evidence. We found moderate certainty of evidence that treatment success was similar with a third-generation cephalosporin compared to penicillin/ampicillin and gentamicin [RR 1.03 (0.93 to 1.13)]. In the non-hospital studies, we found moderate certainty evidence that treatment failure was lower with oral amoxicillin x 7 days + gentamicin x 2 days compared to penicillin and gentamicin x 7 days [RR 0.67 (0.47 to 0.95)].
Conclusion(s): Among the 41 included RCTs, antibiotics regimens varied widely. Most trials were conducted before 2010. There is an urgent need for more rigorous RCTs to determine optimal antibiotic regimens for treating sepsis in the current era of shifting epidemiology and antimicrobial resistance patterns.
.png)
