Infectious Diseases
Session: Infectious Diseases 3
125 - Genomic Characterization of Invasive and Colonizing Antibiotic-Resistant Enterobacterales in Infants
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 125
Publication Number: 125.1875
Publication Number: 125.1875

Nabgha Farhat, MD (she/her/hers)
Pediatric Infectious Diseases Fellow
Ann & Robert H. Lurie Children's Hospital of Chicago
Chicago, Illinois, United States
Presenting Author(s)
Background: Infants are vulnerable to antibiotic-resistant bacterial infections, including extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-E). ESBL-E can asymptomatically colonize the gut of young infants or cause invasive infections leading to prolonged hospitalization, morbidity, and mortality. We hypothesize that ESBL-E that colonize the gut are distinct from those that cause invasive infections.
Objective: Characterize differences between antibiotic-resistant Enterobacterales that cause invasive infections in young infants and asymptomatic stool colonizers.
Design/Methods: Archived ESBL-E isolates from cases of bacteremia in infants less than 12 months of age over the past 10 years were retrospectively retrieved from hospital clinical microbiology labs. Concurrently, Enterobacterales isolates grown on selective MacConkey agar with ceftriaxone were collected from stool of healthy community infants as part of an ongoing observational study “The Acquisition of Resistant Bacteria (ACQUIRE).” Invasive blood isolates were compared to stool colonizing isolates.
Bacterial DNA was extracted using Nextera Prep Kit (Illumina) and sequenced on the MiSeq Platform at Northwestern University Sequencing Core facility. De novo genome assembly was performed using SPAdes. Genomes were annotated using the RAST tool kit. Resistance genes were identified to calculate the frequency of genotypic antimicrobial resistance. A phylogenetic tree was constructed to determine strain relatedness.
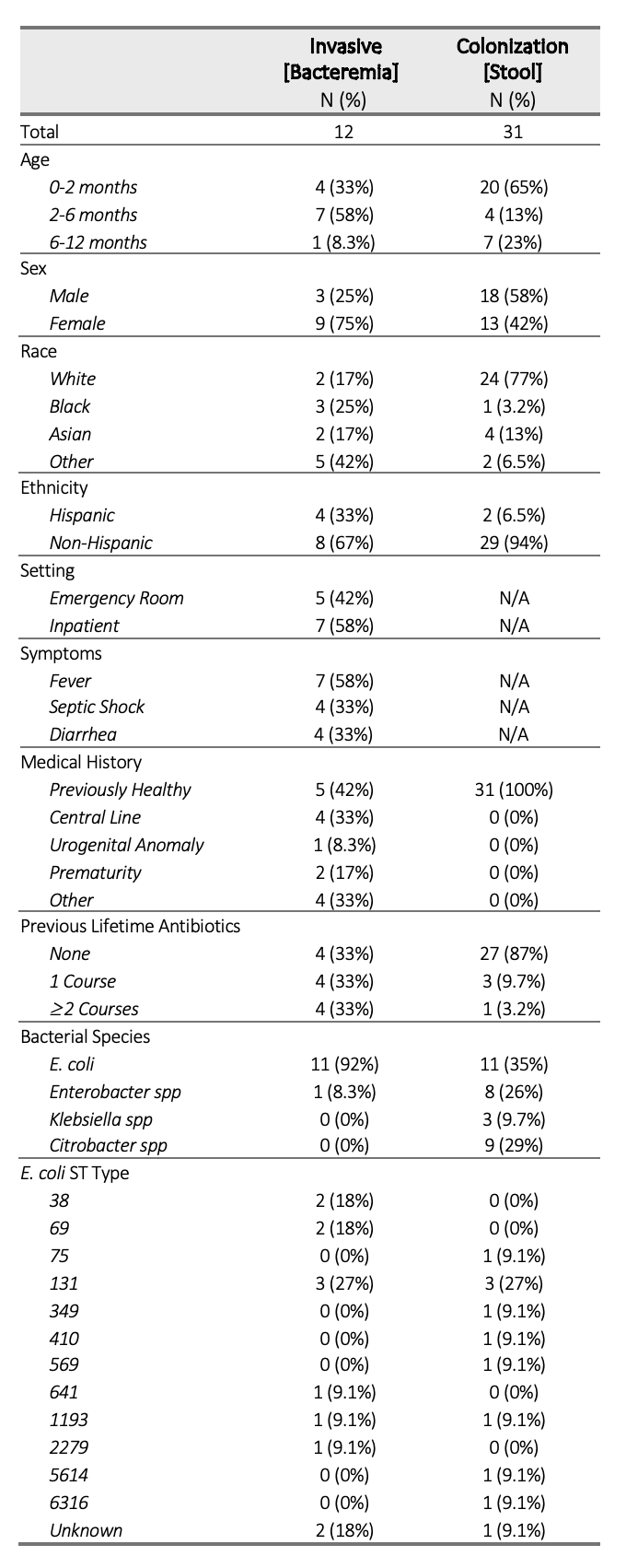
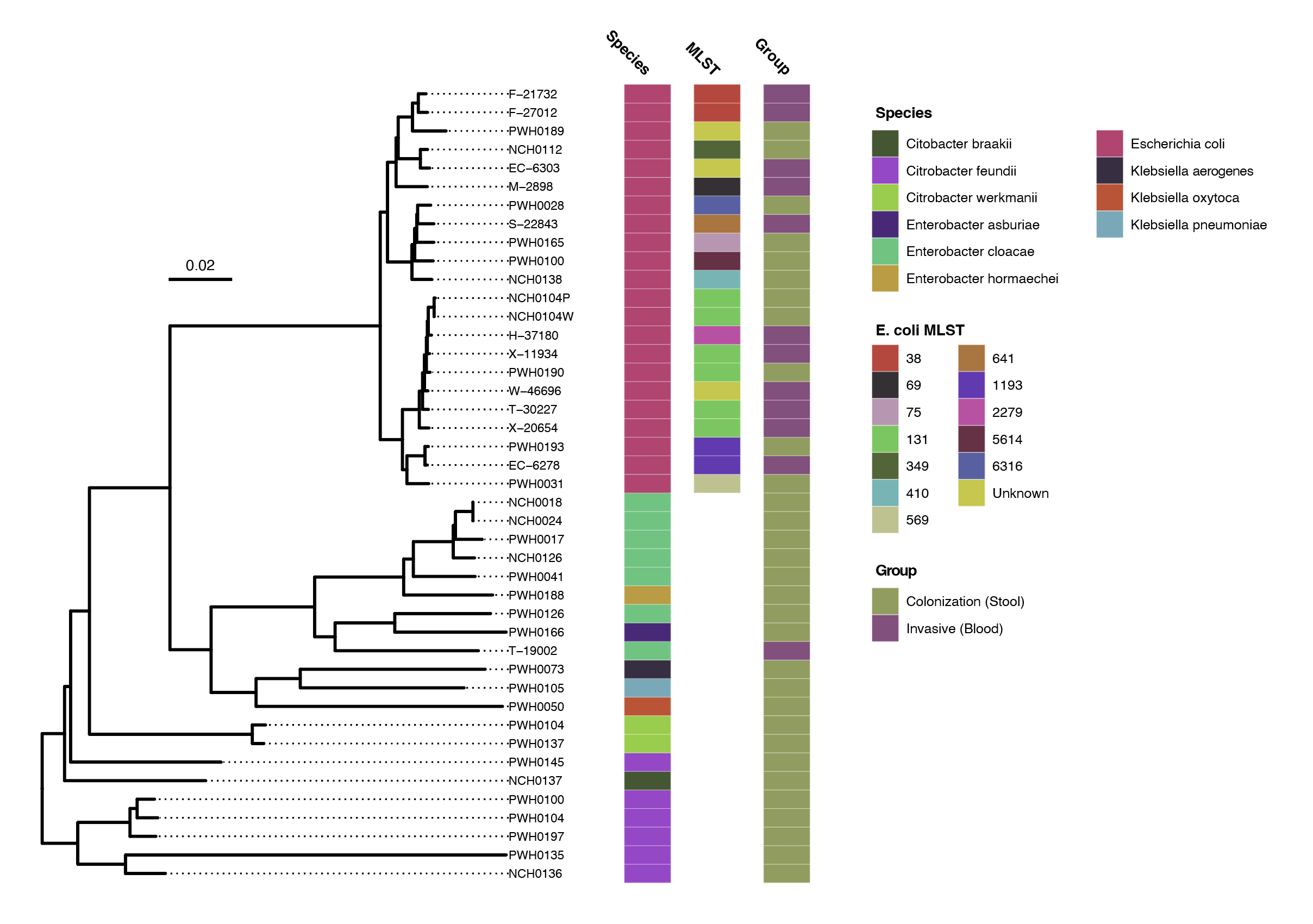
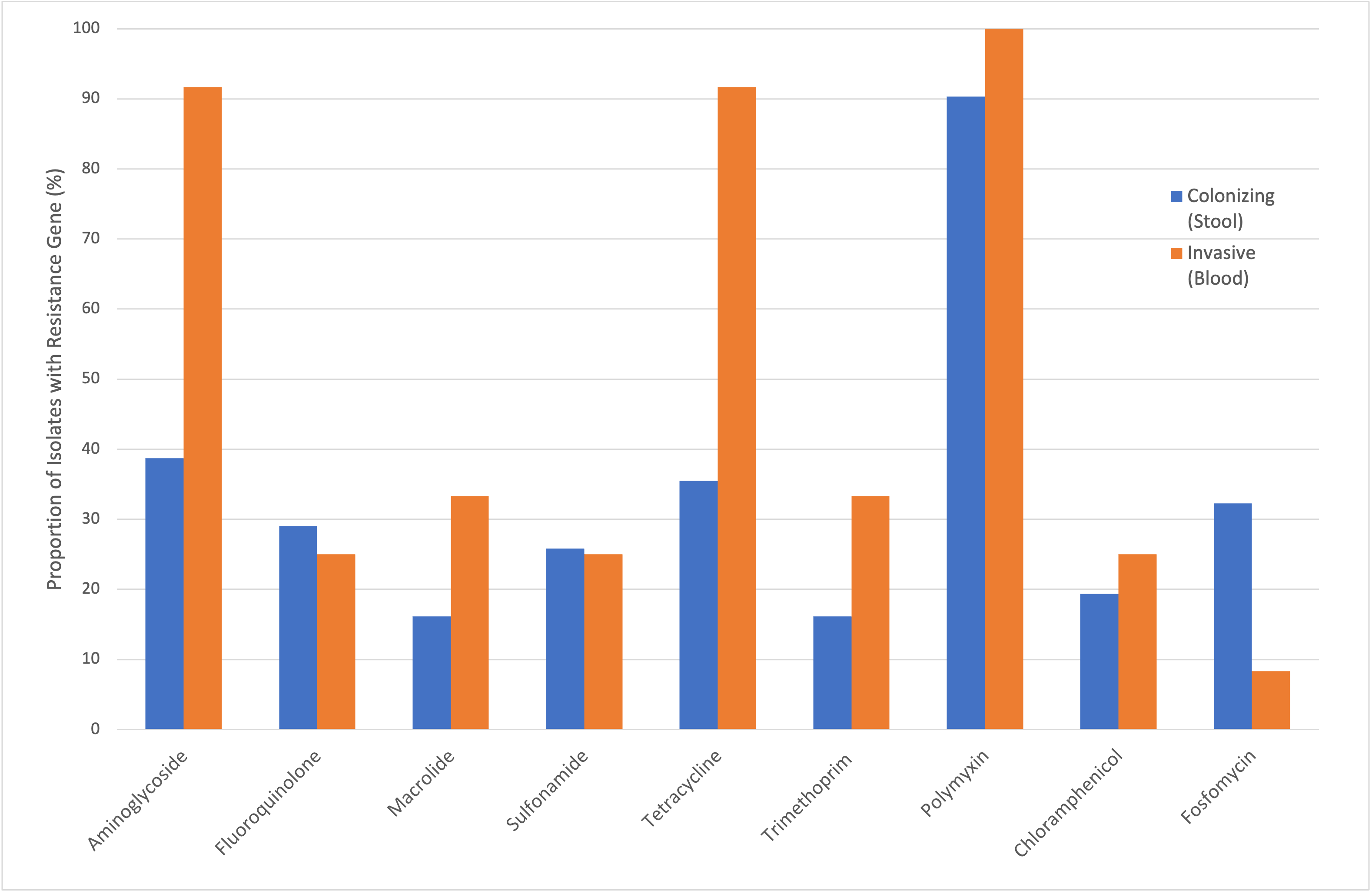
Results: We identified 12 invasive and 31 colonizing antibiotic-resistant Enterobacterales isolates in infants. Characteristics of these isolates are described in Table 1. The E. coli strain ST 131 was most common and observed in both colonizing (27%) and invasive groups (27%). A phylogenetic tree demonstrated no clear clustering patterns of strains in invasive and colonizing groups (Figure 1). The frequency of genes conferring resistance to different antibiotic classes were compared, showing increased proportion of antibiotic resistance in invasive isolates, particularly to aminoglycosides and tetracyclines (Figure 2).
Conclusion(s): While many drug-resistant Enterobacterales species colonize the gut of healthy young infants, bacteremia was disproportionately caused by E. coli. The global pandemic E. coli strain ST131 was found to be both an asymptomatic colonizer and invasive pathogen in U.S. infants. Ongoing cohort enrollment and further analysis comparing key genomic virulence factors may inform the future development of targeted interventions to mediate colonization and reduce invasive infection.