Neonatology
Session: Neonatal Quality Improvement 2
395 - A Quality Improvement Initiative to Optimize Genetic Diagnosis in a Level IV Neonatal Intensive Care Unit
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 395
Publication Number: 395.1921
Publication Number: 395.1921

Alissa M. D'Gama, MD PhD (she/her/hers)
Clinical Fellow
Boston Children's Hospital
Dedham, Massachusetts, United States
Presenting Author(s)
Background: Many infants admitted to Level IV Neonatal Intensive Care Units (NICUs) have underlying genetic disorders, which contribute to substantial morbidity and mortality. Early identification of underlying genetic diagnoses has the potential to impact management and improve outcomes. However, comprehensive genetic testing is often not performed during the NICU admission, leading to delayed or missed genetic diagnoses.
Objective: Our primary aim was to increase the percentage of NICU infants undergoing genetics evaluation who meet criteria for rapid genomic sequencing (rGS) and have rGS sent from 36% to 72% by March 2024. Our secondary aim was to decrease the turnaround time of rGS (from genetics consult to final result) from a median of 18 days to 16 days over this same time period.
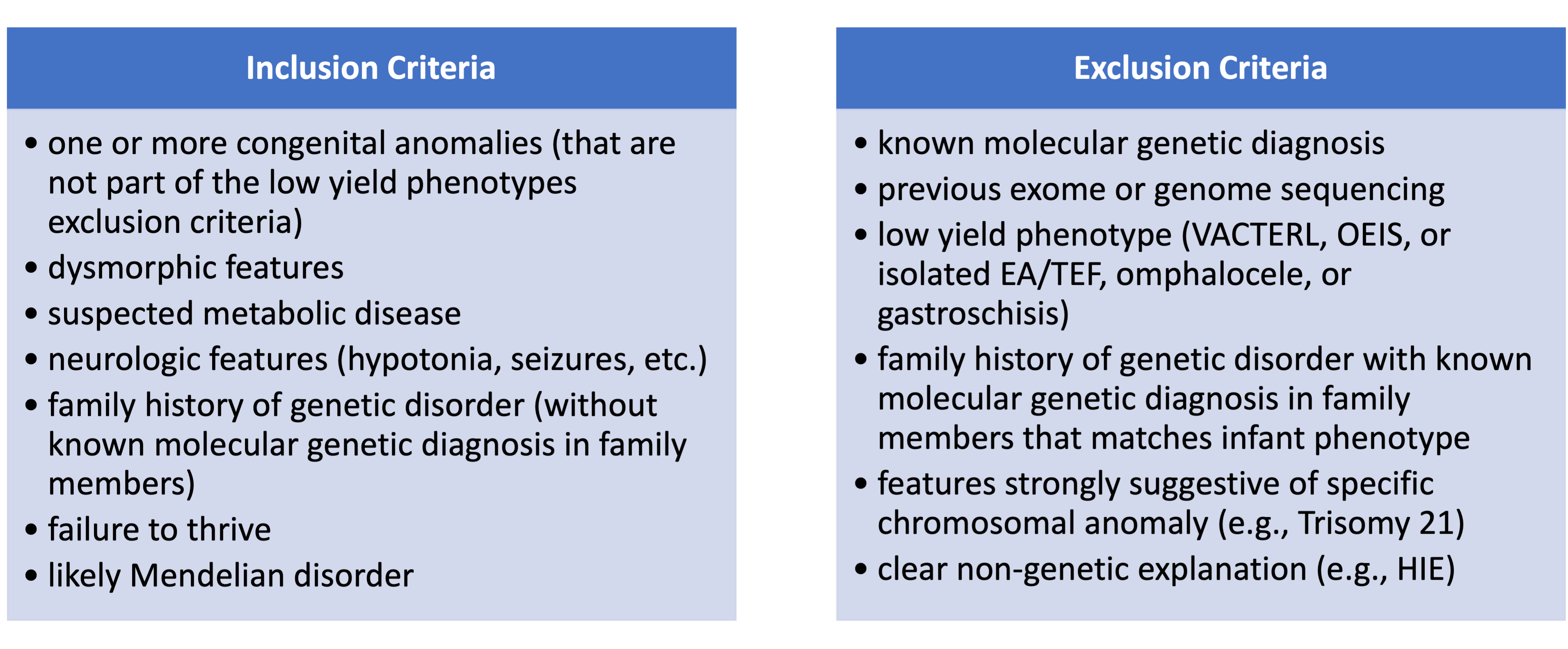
Design/Methods: We established a multidisciplinary Neonatal Genomics Program (NGP) at Boston Children’s Hospital to optimize genomic care for infants with suspected genetic disorders admitted to our Level IV NICU. Baseline data was collected for infants admitted to the NICU and undergoing genetics evaluation from January 2019 – March 2021. This ongoing QI study used the Model for Improvement with sequential PDSA cycles: biweekly review of NICU genetics consults by the NGP to identify infants who qualify for rGS (started April 2021 and ongoing), education of NICU providers on NGP-developed criteria for rGS (Table 1, March 2022), simplified rGS request form (April 2022), step-by-step rGS ordering workflow for NICU staff (May 2022), and refined criteria and re-education of NICU staff (July 2023).
Results: We collected and analyzed data quarterly (Figure 1). For our primary outcome measure, the percentage of qualified infants who had rGS sent increased from 36% pre-intervention to 50% post-intervention thus far (through September 2023). As a balancing measure, the diagnostic yield of rGS sent remained stable at 31% pre-intervention and 33% post-intervention, suggesting that we were not sending unnecessary rGS tests. For our secondary outcome, the median time from genetics consult to rGS result decreased from 18 days pre-intervention to 17.5 days post-intervention.
Conclusion(s): In this ongoing QI study, we have seen an increase in appropriate utilization of rGS for critically ill infants with suspected genetic disorders through proactive identification of these infants and education of NICU staff, with a stable rGS diagnostic yield. We are planning additional PDSA cycles to evaluate maintenance of this finding and to help achieve our intervention goals.
.png)
