Nephrology
Session: Nephrology 3
12 - Quality of life in infant survivors of prenatal serial amnioinfusions in the RAFT trial; a pilot study
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 12
Publication Number: 12.2002
Publication Number: 12.2002

Samhita Vasu (she/her/hers)
Undergraduate Student
Johns Hopkins University
Baltimore, Maryland, United States
Presenting Author(s)
Background: Anhydramnios due to fetal renal failure is associated with lethal pulmonary hypoplasia in the neonatal period. The Renal Anhydramnios Fetal Therapy (RAFT) trial investigates how restoring amniotic fluid via serial amnioinfusions may promote fetal lung development, potentially enabling survival. However, infants who survive have end stage kidney disease and require intensive, family-provided care even after their initial discharge home on chronic peritoneal dialysis.
Objective: Our objective was to cross-sectionally assess quality of life (QOL) for infants in the RAFT trial who survived to hospital discharge and their families.
Design/Methods: Study participants had confirmed anhydramnios due to either bilateral renal agenesis or other cause of fetal renal failure (e.g. multicystic dysplastic kidneys, severe lower urinary tract obstruction). Families were administered validated PedsQL™ standardized questionnaires which included the Family Impact Module Parent Report and the Infant Scales Parent Report for Infants. Functional domains included physical, emotional, social and cognitive. Parents rated degree of agreement with statements indicating problems in each domain on a scale of 0 (never) to 4 (almost always).
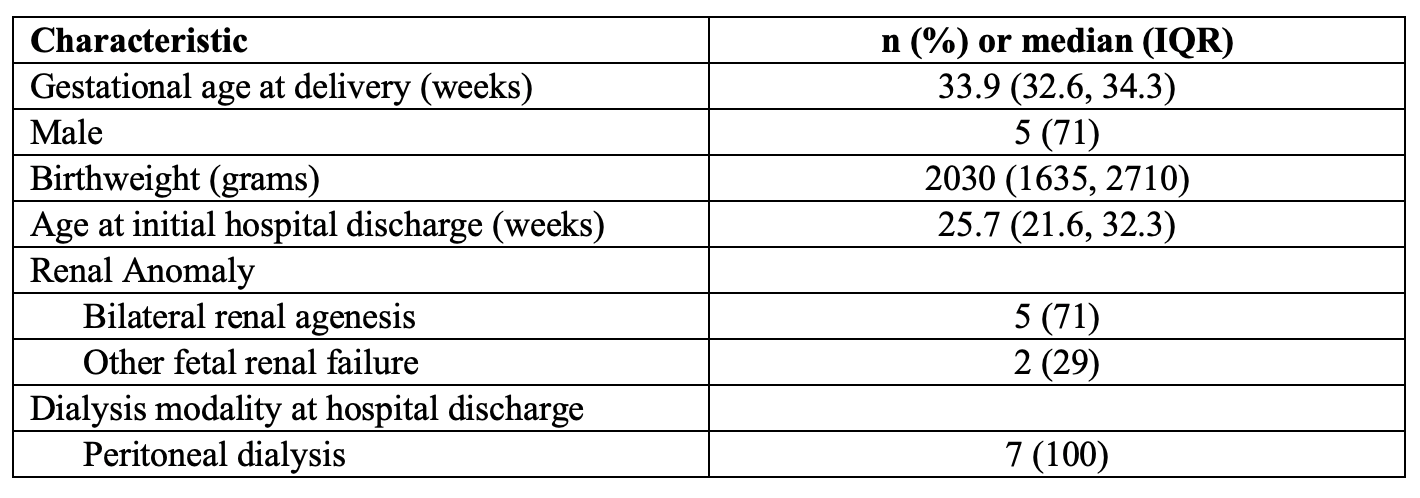
Results: Infants had a median (IQR) gestational age at delivery of 33.9 (32.6, 34.3) weeks and length of initial hospitalization was 25.7 (21.6, 32.3) weeks, and 71% had bilateral renal agenesis. 75% of parents reported that family activities sometimes or almost always took more time and effort, and that others sometimes or almost always do not understand their family situation. 100% of parents reported that they often, sometimes, or almost always worry about their child’s future and about the side effects of their child’s medications. 87.5% of the parents reported often, sometimes, or always feeling anxious. 87.5% of parents reported their children having low energy sometimes or often, sometimes crying and fussing when left alone, feeling cranky, and having gas. 100% of parents responded “never” or “almost never” to infant problems making eye contact with a caregiver.
Conclusion(s): Parents of infants with end stage kidney disease after RAFT trial participation report stress, fatigue and anxiety within the family along with fussiness in infants. Further investigation is required to assess how these reported QOL indicators compare to families and infants with other chronic medical conditions and to otherwise healthy children. Repeated QOL assessments are planned at 6-month intervals to capture changes over time.