Clinical Bioethics
Session: Clinical Bioethics
432 - Standardizing Clinical Ethics Consultation Documentation
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 432
Publication Number: 432.2013
Publication Number: 432.2013

Sunny Jeong, BScN, RN, MBE (she/her/hers)
Pediatric Ethics Fellow
Children's Mercy Hospitals and Clinics
Kansas city, Missouri, United States
Presenting Author(s)
Background: Clinical ethics consultations (CECs) require documentation. There is a paucity of literature and resources to guide clinical ethicists in documenting consults. High quality documentation encourages transparency between patients and clinicians, while keeping interdisciplinary team members knowledgeable about evolving care plans. However, there are discrepancies in the details captured in the CEC notes and accompanying recommendations.
Objective: To identify how CECs are documented in the medical record and assess whether there is consistent inclusion of relevant information.
Design/Methods: A retrospective review of CECs conducted at a free-standing children’s hospital from January 2018 to June 2023 was conducted. CECs were captured by cross-referencing Ethics Committee (EC) meeting minutes with electronic paging notifications and patient charts. Charts were reviewed for completion of a CEC documentation, and if present, the documentation was reviewed for presence of key details including mechanism of consultation, person requesting consultation, statement of ethical question(s), provision of recommendations, and evidence-based citations to justify recommendations.
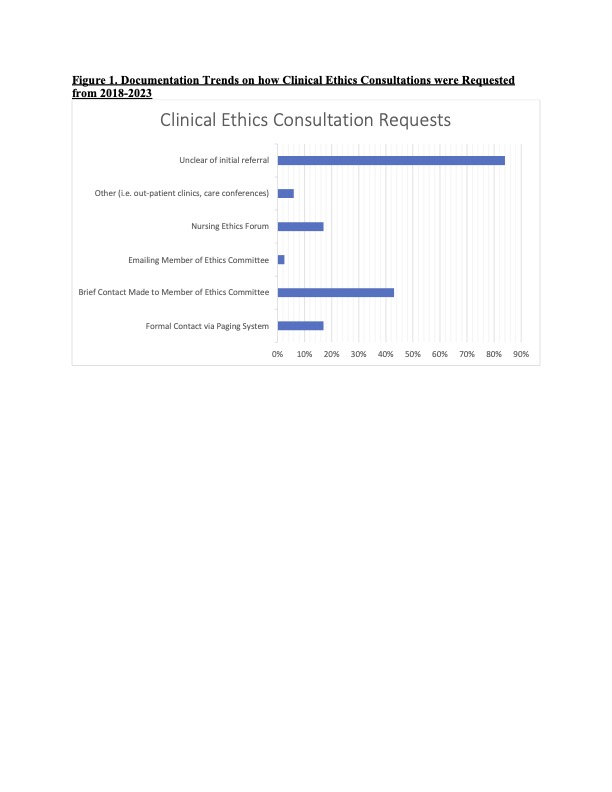
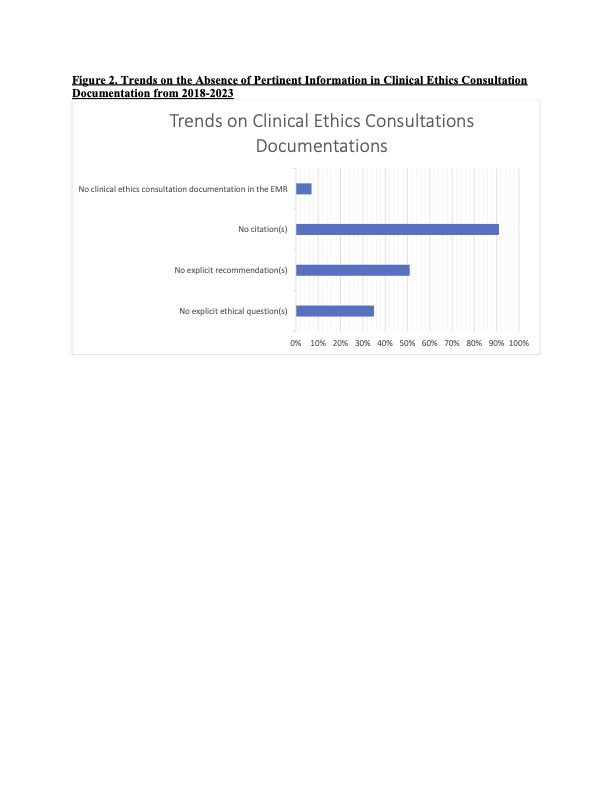
Results: A total of 255 CECs were identified over the study period. Documentation of CECs varied considerably in length and quality. 18% (n=45) of the consults did not clearly document how the initial request was made or whether the requesting person wished to remain anonymous. The use of a formal paging system was noted in only 16% (n=40) of CECs, while the majority (67%; n=170) of CECs were requested informally via emailing/calling EC members and use of hospital ethics forums (Figure 1). Explicit ethical question(s) were absent in 35% (n=89) of the notes; 51% (n=131) did not provide recommendations; and 91% (n=231) did not provide citations supporting their ethical analysis and recommendations. Documentation in the medical record was absent in 7% (n=17) of CECs (Figure 2).
Conclusion(s): There is a need to standardize how CECs are documented to ensure timely follow-ups with key stakeholders, while respecting those who wish to stay anonymous; and to ensure meaningful information and recommendations are appropriately documented. Standardization of CEC documentation could better support clinicians: 1) identify what information is pertinent to include; 2) allow ECs to determine priorities based on clinical ethics needs; 3) elucidate organizational ethics considerations emerging from CECs; 4) help new ethics fellows, consultants, and EC members learn from past CECs; and 5) keep an organized database for reference when similar cases arise.