Neonatology
Session: Neonatal Nephrology/AKI 1
36 - Biomarkers of early acute kidney injury in critical neonates (BEACoN): a pragmatic embedded study
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 36
Publication Number: 36.2040
Publication Number: 36.2040

Kim T. Vuong, MD, MPH (she/her/hers)
Pediatric Nephrology Fellow
Baylor College of Medicine
Houston, Texas, United States
Presenting Author(s)
Background: Neonatal acute kidney injury (AKI) is a potentially preventable mortality, morbidity, chronic lung disease, and chronic kidney disease risk factor with incidence of 30% in the first 7 days of life (DOL). The current definition of neonatal AKI uses serum creatinine (SCr) and urine output (UOP) criteria which have several limitations. While AKI is potentially reversible, SCr lags by 48-72h. Several candidates for early biomarkers for AKI, such as serum Proenkephalin A 119–159 (PENK) and Cystatin C (CysC), are of interest for timely identification of AKI to allow for successful interventions, but neonates in the intensive care unit (ICU) are a challenging population to study.
Objective: Our primary aim was to compare biomarkers (PENK and CysC) in critically ill neonates with and without early AKI (within 7 DOL) using scavenged samples. We hypothesize that these biomarkers will be significantly higher in neonates with AKI compared to neonates without AKI (defined by KDIGO SCr and UOP criteria).
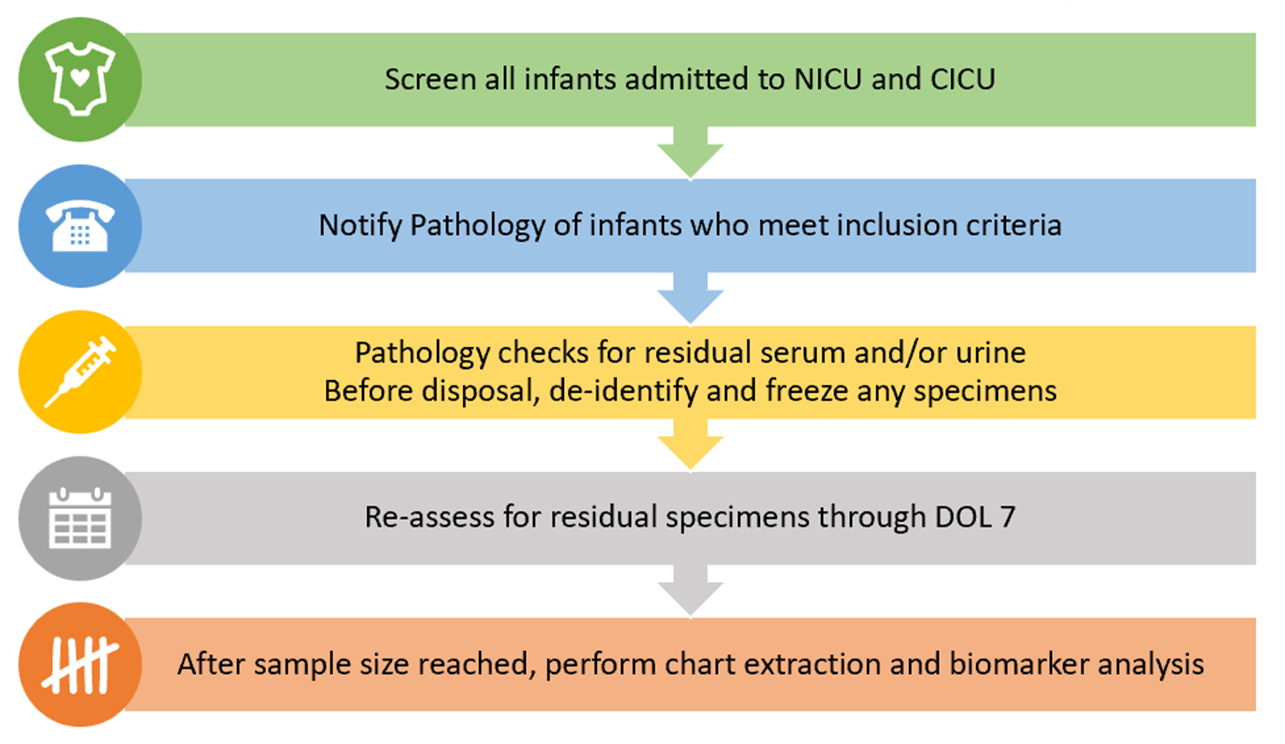
Design/Methods: This is a pragmatic study at a quaternary care pediatric hospital with 118 Level III/IV neonatal and 54 cardiac ICU beds. This study is BCM IRB approved and designed as a scavenging program using residual biospecimens collected for clinical purposes. While reducing iatrogenic anemia, this opportunistic approach will prognostically enrich our sample. We screened all hospitalized neonates for inclusion by high-risk criteria for AKI (hypoxic ischemic encephalopathy, low birth weight, prematurity, suspected sepsis, nephrotoxin exposure, intubated, vasoactives, and congenital heart defects) and exclusion (>14 DOL, not expected to survive >24h, mortality < 48 HOL, known congenital anomalies of the kidney and urinary tract, or renal replacement therapy before labs were available). Residual specimens through DOL7 were frozen (Figure 1).
Results: To date, 243 patients have been screened, 202 met inclusion, and 147 were cross-referenced for residual specimens. Study workflow was modified successfully to overcome low scavenged volume, unmeasured UOP, and lack of 2 SCr to determine AKI status. 143 patients with adequate residual specimens were ultimately included in this interim analysis. Of these, 64 (45%) had 2 biomarkers analyzed (PENK and CysC) and 79 (55%) had 1 biomarker analyzed (CysC).
Conclusion(s): This opportunistic approach of using residual clinical samples is a feasible design that allows investigators to tap into unused specimens in this vulnerable population of critically ill neonates. Workflow modifications can be successful in optimizing patient recruitment.