Nephrology
Session: Nephrology 4
16 - A Comparison of Themes from Concept Elicitation on Fluid Overload Between Older Children and Caregivers of Younger Children in "Preparing a Clinical Outcomes Set for Nephrotic Syndrome" (Prepare-NS)
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 16
Publication Number: 16.2043
Publication Number: 16.2043

Eloise Salmon, MD
Clinical Assistant Professor
University of Michigan Medical School
Ann Arbor, Michigan, United States
Presenting Author(s)
Background: Individuals with nephrotic syndrome (NS) experience fluid overload (FO) which can impact health-related quality of life (HRQOL). In partnership with the Food and Drug Administration (FDA), PREPARing a clinical outcomes assessment set for Nephrotic Syndrome (Prepare-NS) aims to create and validate clinical outcome assessments (COAs) of FO in NS for use in drug development. The COA set will include an observer reported outcome (ObsRO) for young children and a patient reported outcome (PRO) for older children and adults.
Objective: To compare key themes from concept elicitation interviews for ObsRO and PRO that capture important aspects of fluid overload in NS and its impact on function in children.
Design/Methods: For ObsRO, parents/guardians caring for a child age 2-11 with Focal Segmental Glomerulosclerosis (FSGS), Minimal Change Disease (MCD), Childhood-onset Nephrotic Syndrome Not Biopsied (Childhood), Membranous Nephropathy (MN), or IgM Nephropathy who has current or history of NS-associated edema within the past 3 months volunteered via study website to complete an interview. Following informed consent, concept elicitation interviews occurred via Zoom or phone and discussed how the child’s “NS” impacts functioning, with a specific focus on observable experiences. Interview transcripts were coded in NVivo by two independent raters according to established standards for qualitative analysis. For PRO, children 8 or older could complete an interview with parental consent and personal assent. Parents/guardians did not complete an ObsRO interview if their child completed a PRO interview.
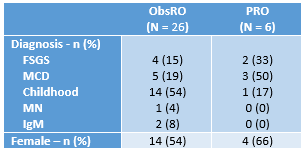
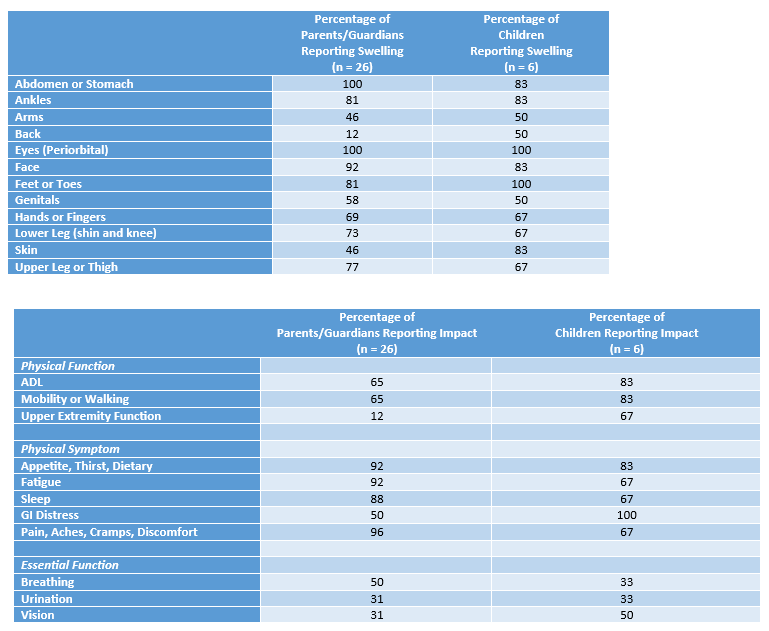
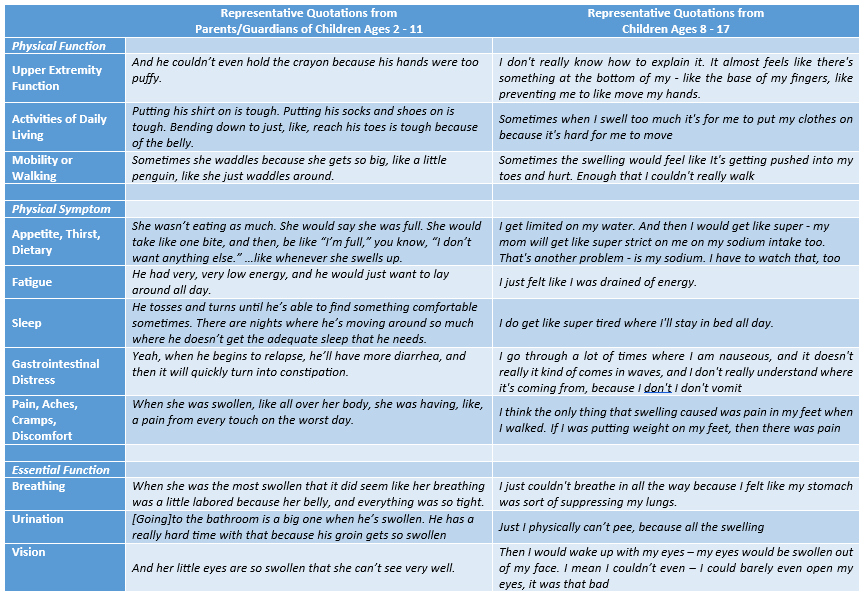
Results: Figure 1 provides demographic information for the impacted children. Figure 2 shows the percentage of parents/guardians of young children and the percentage of older children reporting swelling in specific body parts and particular impacts of FO. Figure 3 highlights illustrative quotes from parents/guardians of young children and from older children.
Conclusion(s): Concept elicitation interviews about FO in NS uncovered similar experiences from caregivers of young children and from older children. The next phase of Prepare-NS is development and validation of an ObsRO and a PRO inclusive of items likely to be modifiable by pharmacologic therapy to create a harmonized COA set for use in drug development.