Neonatology
Session: Neonatal Hematology & Bilirubin Metabolism
462 - The impact of red blood cell donor sex on recipient morbidity and mortality in transfused extremely preterm infants: A Systematic Review and Meta-analysis.
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 462
Publication Number: 462.1169
Publication Number: 462.1169
- MS
Michael J. Stark, BSc(Hons) MBChB FRCP FRACP PhD (he/him/his)
Deputy Director
The Robinson Research Institute, University of Adelaide
North Adelaide, South Australia, Australia
Presenting Author(s)
Background: The potential for packed red blood cell (PRBC) transfusion-related adverse outcomes is an area of growing concern in neonatal medicine. Attention has recently shifted to the influence of donor factors on adverse post-transfusion outcomes.
Objective: We aimed to compare the impact on preterm mortality and morbidity (bronchopulmonary dysplasia, intraventricular haemorrhage, necrotising enterocolitis, or retinopathy of prematurity) of receiving a PRBC transfusion from only male donors compared to receiving any red blood cell transfusion from a female donor.
Design/Methods: A systematic search of the literature was conducted using Medline, Embase, Cumulative Index to Nursing and Allied Health Literature, PubMed, Web of Science, Cochrane Database of Systematic Reviews. The Data bases were last searched in July 15, 2023. Studies were included if they were randomised, quasi-randomised, interrupted time series, controlled before and after, and cohort studies (retrospective and prospective). Studies needed to compare PRBC transfusion from only male donors versus PRBC transfusion from any female donor in preterm infants < 32 weeks’ gestation or very low birth weight infants < 1500g were eligible for inclusion. Two authors independently extracted data, assessed risk of bias, and certainty of evidence. Risk of bias was assessed using the Cochrane Risk of Bias toll for randomised trials and the Non-Randomised Studies of Interventions Tool (ROBINS-1) for observational studies.The outcomes of interest were mortality, moderate to severe bronchopulmonary dysplasia, necrotising enterocolitis stage 2 or greater, retinopathy of prematurity stage 3 or greater in either eye, intraventricular haemorrhage grade 3 or 4.
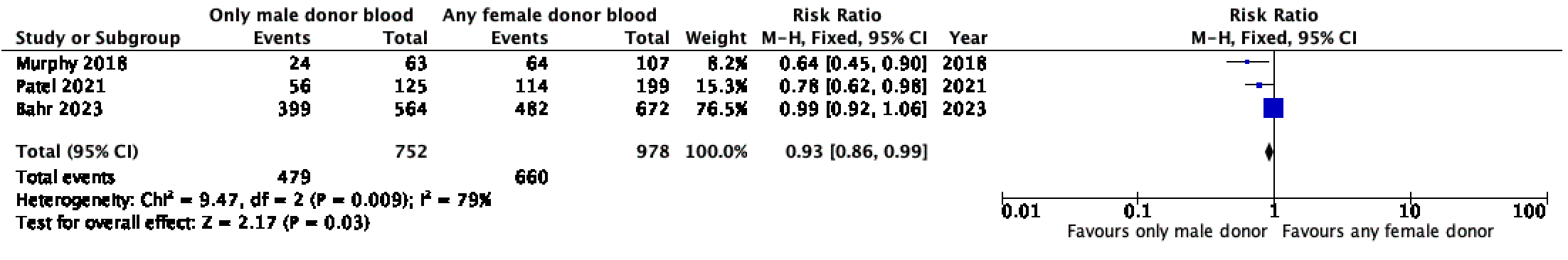
Results: Three retrospective observational cohort studies met inclusion criteria (1730 newborns). These studies showed that exposure to PRBC transfusion from only male donors decreased the likelihood of BPD (RR (95% CI) 0.71 (0.54 – 0.94), p=0.02) ROP (RR (95% CI) 0.71 (0.55 – 0.91), p=0.007) and a composite of mortality and morbidity (RR (95% CI) 0.93 (0.86 – 0.99), p=0.03) when compared to any PRBC transfusion from a female donor.
Conclusion(s): Certainty of evidence was very low for all outcomes. Among preterm infants < 32 weeks’ gestation or very low birth weight infants < 1500g birth weight, who received one or more red blood cell transfusion during their initial hospitalisation, exposure to any female donor blood was associated with increased risk of bronchopulmonary dysplasia, retinopathy of prematurity and a composite outcome of mortality and /or significant neonatal morbidity.
.png)
.png)