Neonatology
Session: Neonatal Infectious Diseases/Immunology 1
589 - Fetal Tissue-derived Enteroids as a Novel Model to study GBS pathogenesis
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 589
Publication Number: 589.528
Publication Number: 589.528

Alexia N. Pearah, MS (she/her/hers)
Research Associate
University of South Florida
Tampa, Florida, United States
Presenting Author(s)
Background: Group B Streptococcus (GBS) is a leading cause of neonatal sepsis globally. GBS colonization in the gastrointestinal tract is a critical precursor to late-onset disease in exposed neonates. Studies of underlying pathogenesis rely on animal models with limited human relevance or in vitro models that rely on transformed, adult human intestinal cell lines which do not replicate the responsiveness of the neonatal epithelium and the complexity of the intestinal environment. Previous studies using human fetal tissue (HFT)-derived enteroids to model interactions with enteric pathogens have yielded valuable insights. However, this has yet to be explored with GBS.
Objective: We aimed to develop a model of GBS-exposed (HFT)-derived, apical-out enteroids to study GBS interactions in an immature human intestinal environment.
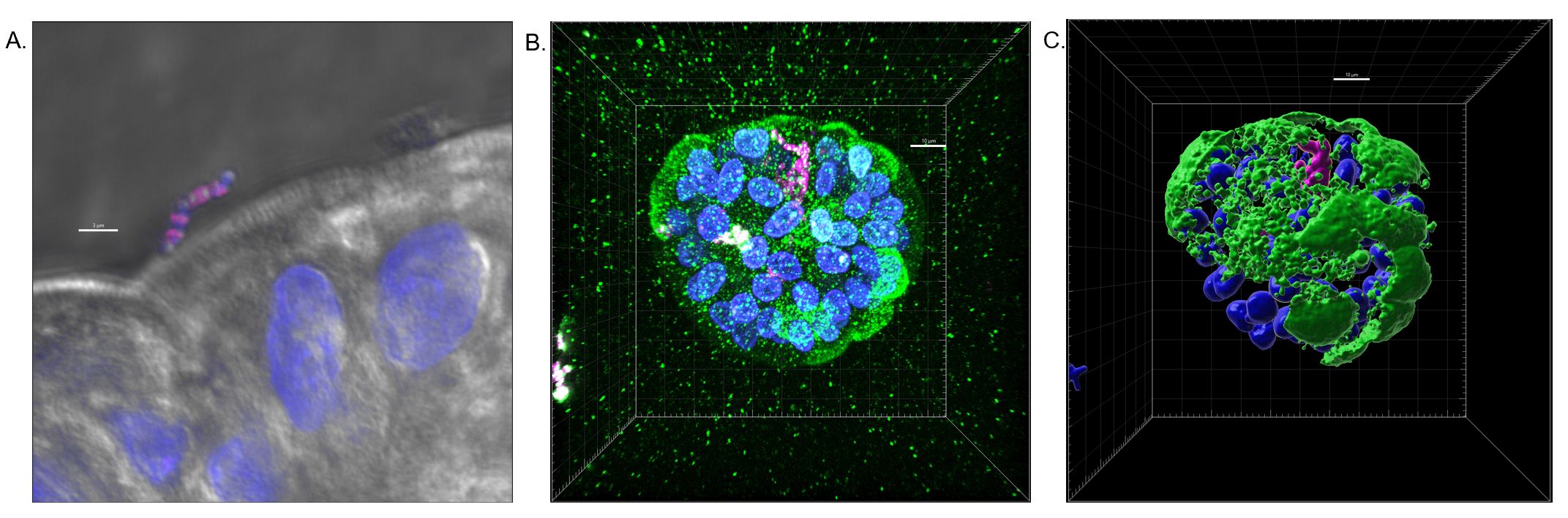
Design/Methods: The enteroid cell line was cultivated from human fetal tissue samples obtained from Birth Defects Research Laboratory (University of Washington). The polarity of the epithelial cells was reversed using 5mM EDTA to generate apical-out enteroids that were seeded on a low attachment plate. On day 3 of apical exposure establishment, enteroids were infected with GBS COH-1 serotype III ST-17 with optical density at 600 nm (OD600) of 0.4 (1 x 108 CFU/mL) and 0.8 (1 x 109 CFU/mL). Enteroids were exposed to GBS added directly to the media for 2 hours at 37°C, centrifuged and then washed with phosphate buffered saline. Samples were fixed and stained with commercially available antibodies directed against villin and GBS respectively with appropriate fluorescently-labeled secondary antibodies for visualization using laser scanning confocal microscopy and analyzed using the Imaris software.
Results: Our image analysis demonstrates that GBS firmly attaches to apical surfaces of HFT-derived enteroids and in some cases, translocation through the intestinal epithelial barrier.
Conclusion(s): HFT-derived enteroids are a useful tool for the study of GBS-intestinal cell interactions in the immature host. Further examination of differential intestinal cell types with staining of Paneth cells, goblet cells, enteroendocrine cell marker, and tight junction proteins will reveal site-specific preferences for GBS adhesion and invasion. Our data demonstrates that fetal tissue-derived enteroids can serve as a novel model for studying GBS pathogenesis in the intestinal epithelium, thus allowing for the development of new and effective therapeutics.