Neonatology
Session: Neonatal Clinical Trials 1
463 - Feasibility and Safety of Autologous Cord Blood Derived Cell Administration in Extremely Preterm Infants: A World First Study
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 463
Publication Number: 463.1326
Publication Number: 463.1326

Lindsay Zhou, MBBS (he/him/his)
Neonatologist
Monash University
Clayton, Victoria, Australia
Presenting Author(s)
Background: Preterm brain injury continues to be a common complication of preterm birth, especially in extremely premature infants. Umbilical cord blood-derived cells (UCBCs) are increasingly being evaluated for their neuroprotective and neuro-reparative properties in perinatal brain injury in preclinical and clinical studies. There remains a paucity of information on the feasibility and safety of autologous UCBC administration in extremely premature infants.
Objective: To evaluate feasibility of sufficient cord blood collection, UCBC extraction, and subsequent safety of intravenous UCBC administration in extremely preterm infants.
Design/Methods: A single-center safety and feasibility study (CordSaFe, ACTRN12619001637134) of preterm infants born < 28 weeks’ gestation. Cord blood was collected at birth and if sufficient volume obtained, UCBCs were harvested, characterized (total nucleated cell count (TNC), mononuclear cell count (MNC), CD34+ cells) and cryopreserved. Infants were then infused autologous UCBCs intravenously at a dose of 25–50 million MNCs/kg in postnatal week two. Primary outcomes included feasibility defined as sufficient cord blood at collection (>7 mL) and UCBCs following processing (>25 x 10e6 TNCs/kg and safety demonstrated by lack of adverse events directly related to UCBC administration. Secondary outcomes included neonatal morbidities until discharge as compared to a contemporary cohort of preterm infants.
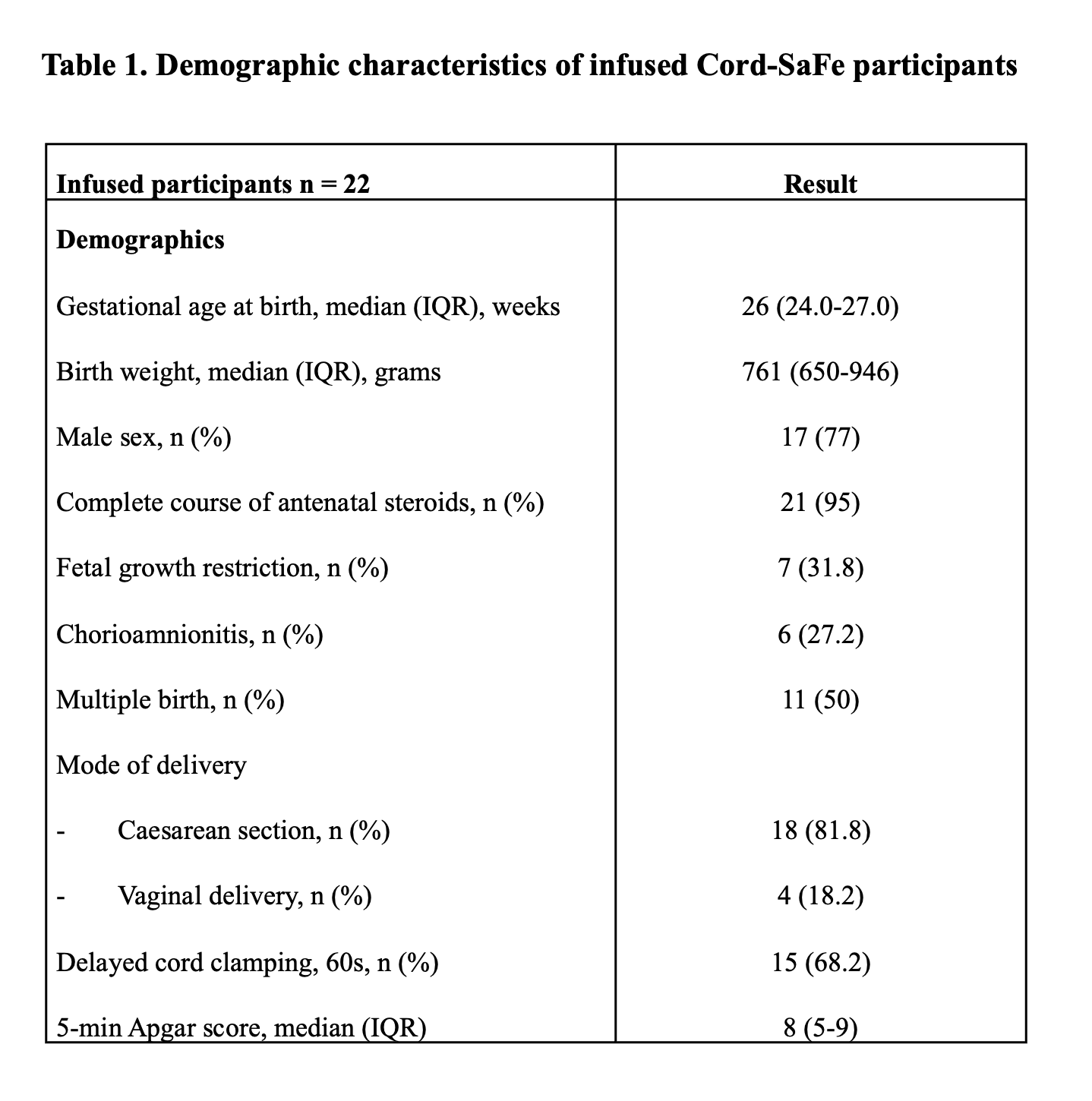
Results: Forty-eight preterm infants were recruited before birth, 41 UCB collections were attempted, and feasibility of sufficient UCB collection/ UCBC extraction was demonstrated in 35 (85%) infants. GMP grade cells were available for infusion in 29 (70%) of infants. Median (IQR) TNCs collected was 123.9 (67.2-191.6) x 10e6/kg, MNCs 60.5 (38.3-106.1) x 10e6/kg and CD34+ cells 1.28 (0.72-2.04) x 10e6/kg. 22 infants with median (IQR) completed gestation of 26 (25-27) weeks, and median (IQR) birth weight 748 (645-981) grams were administered cells (mean (SD) dose of 44.4 (18.2) x10e6 MNCs/kg) at 12-14 days of life with no serious adverse events noted. The incidence of major neonatal morbidities in infused infants was similar to a contemporary cohort of non-infused infants (n=75). Demographic and UCBC data are listed in Table 1 and Table 2. Neuro-developmental follow-up till 2 years corrected age is ongoing.
Conclusion(s): For the first time, we have confirmed UCBC collection is feasible in extremely preterm infants in up to 80% cases, and autologous administration of UCBCs is safe. This paves the way for a randomized controlled trial of UCBC therapy in the extremely preterm infant population.
.png)
