Emergency Medicine
Session: Emergency Medicine 3: Serious Bacterial Infections
86 - Improving Identification and Management of Infants at Risk for Herpes Simplex Virus
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 86
Publication Number: 86.1647
Publication Number: 86.1647

Dominique Chevalier, MD (she/her/hers)
Assistant Professor
University of Utah School of Medicine
Midvale, Utah, United States
Presenting Author(s)
Background: Infantile herpes simplex virus (HSV) infection predominantly affects infants < 29 days old (88% of cases) and is associated with high mortality and morbidity when not recognized and treated in a timely fashion. Concern for ‘missing’ this relatively rare and potentially devastating disease results in over-evaluation and treatment, which incur risks. In our center, among infants ≤ 90 days, we identified potential performance and knowledge gaps, with an unacceptably high rate of HSV testing in infants 29-90 days old, particularly in the emergency department (ED).
Objective: To standardize the identification, evaluation and treatment of infants at highest risk of HSV, and reduce testing and treatment of infants 29-90 days old, by at least 90% within 10 months of implementation.
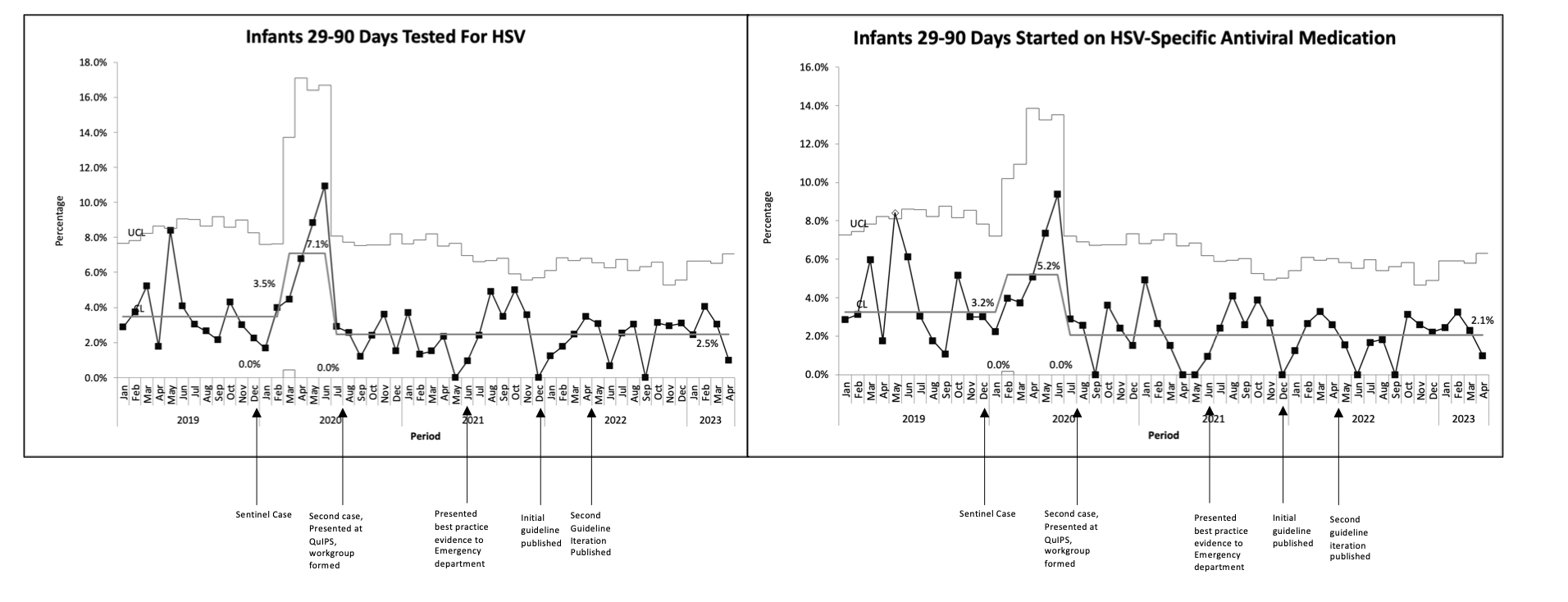
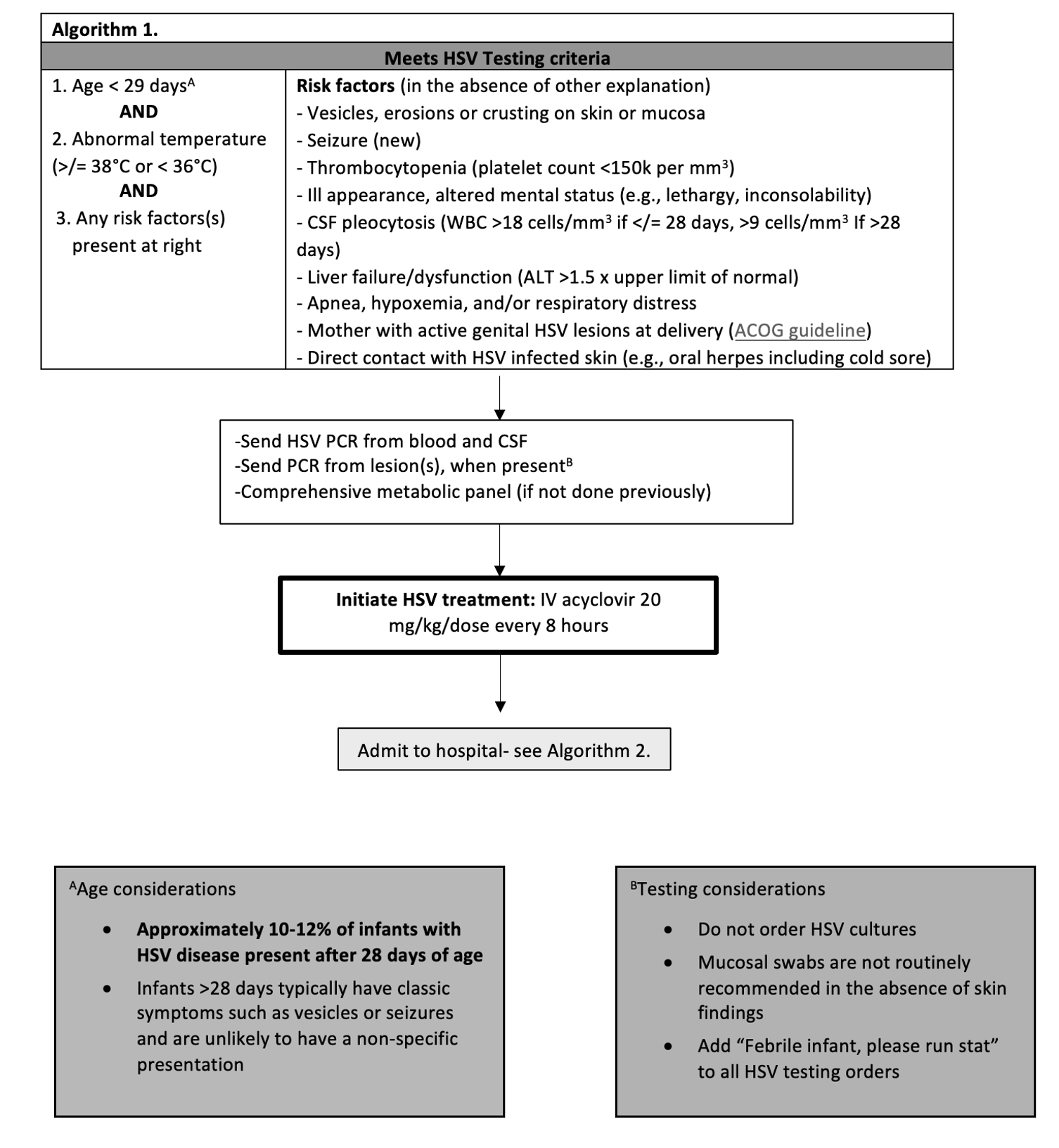
Design/Methods: This was a single-center retrospective, quality improvement study including infants 29-90 days of age who underwent any HSV testing between 1/2019 and 4/2023, excluding NICU patients. A multi-disciplinary workgroup developed and implemented an evidence-based clinical decision support tool (CDST) and treatment guideline. The process underwent several iterative improvement cycles. The main outcome measure was the proportion of infants 29-90 days old who underwent any HSV testing during their ED visit. Additionally, we measured parenteral HSV-specific antiviral medication initiation in the ED among infants 29-90 days old. The balancing measure included patients presenting within 14 days of the initial encounter and subsequently diagnosed with HSV. Statistical process control charts identified process variation using Western Electric rules.
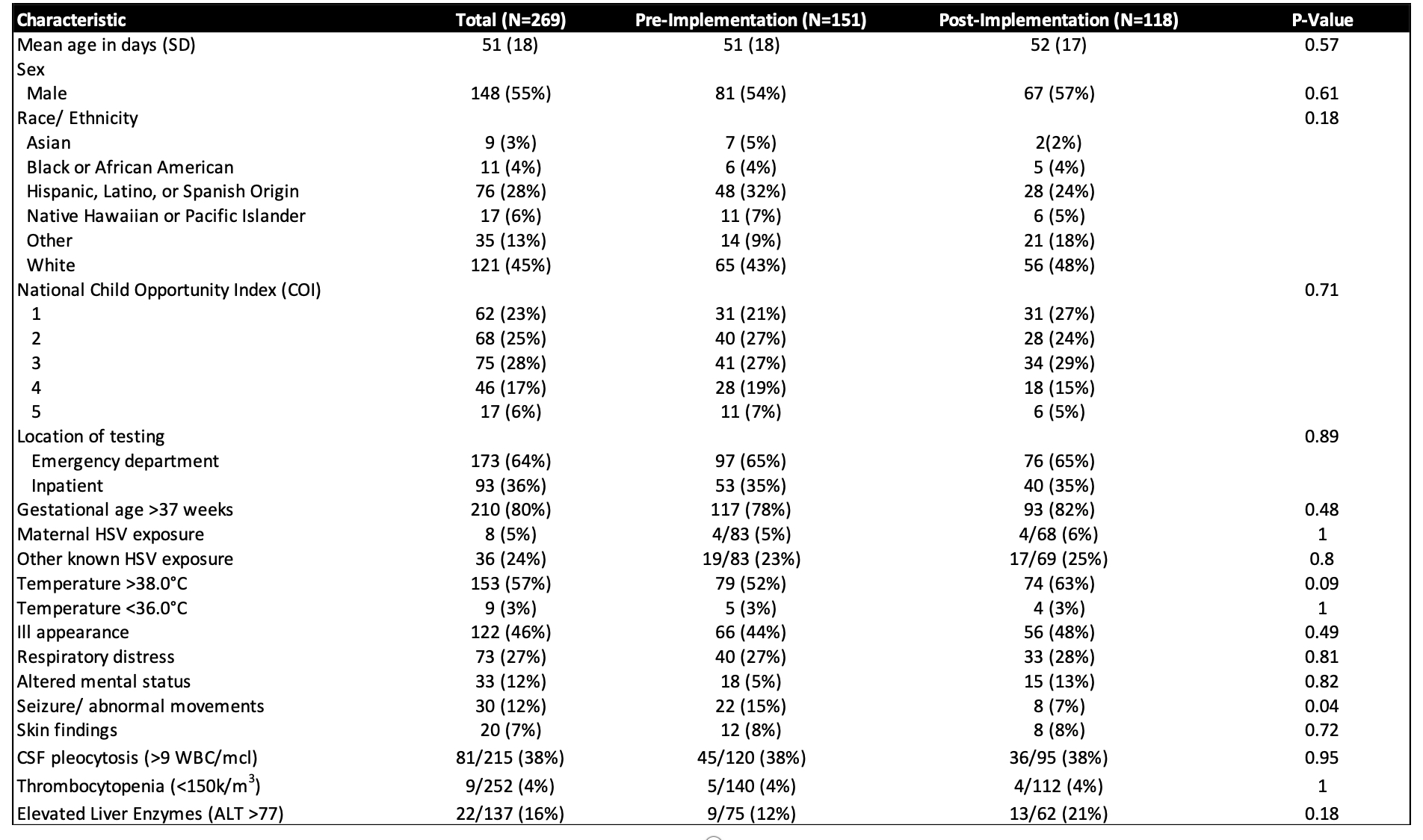
Results: Of the 269 infants who met inclusion criteria, the majority (64%) underwent testing and treatment in the ED. There were 151(56%) and 118(44%) infants in the pre- and post-implementation groups, respectively. The groups were similar, with the exception of more patients in the pre-implementation group presenting with seizure/abnormal movements. Following interventions, the proportion of 29-90 days old infants tested in the ED for HSV decreased 65%, from 7.1% to 2.5% within 2 months, which has been sustained over the subsequent 33 months. Parenteral HSV-specific antiviral medication initiation in the ED followed a similar pattern. No patients returned within 14 days and were subsequently diagnosed with HSV.
Conclusion(s): HSV testing and treatment of infants 29-90 days of age in the ED decreased by 65% following multiple iterative changes, without an apparent increase in missed cases of HSV.