Neonatology
Session: Neonatal Pulmonology - Clinical Science 2: Lung Imaging, Lung Function
198 - Normal saline boluses adversely affect respiratory physiology in extremely premature infants
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 198
Publication Number: 198.3339
Publication Number: 198.3339
- AS
Arvind Sehgal, PhD (he/him/his)
MONASH UNIVERSITY
MELBOURNE, Victoria, Australia
Presenting Author(s)
Background: Extremely premature infants are at a higher risk of developing circulatory impairments in the first few weeks of life. Administration of normal saline bolus is a common practice to manage hypotension. As a crystalloid, a substantial proportion might leak into the interstitium; most consequently the lungs in the preterm cohorts, putatively affecting ventilation.
Objective: The objective of this study was to evaluate the evolution of pulmonary physiology when normal saline bolus was administered to mechanically ventilated extremely premature infants.
Design/Methods: We analysed ventilator mechanics data in infants managed by conventional ventilation and administered normal saline bolus. Infants >7 days old or self-ventilating/on other modes of ventilation were excluded. We included infants ≤28 weeks gestational age (GA) being administered saline bolus (10ml/kg over 60 minutes) for reasons determined by the clinical team. Data included 30 minutes pre-bolus, 60 minutes during the bolus, and 30 minutes post-bolus. Infants were on volume-controlled PC-AC Drager Babylog VN500 ventilator. No change was made to ventilator settings except nurse controlled change in FiO2 to maintain saturations within the nominated range (91-95%). The set volume guarantee in the cohort was median 5 ml/kg (range 4.5-5).
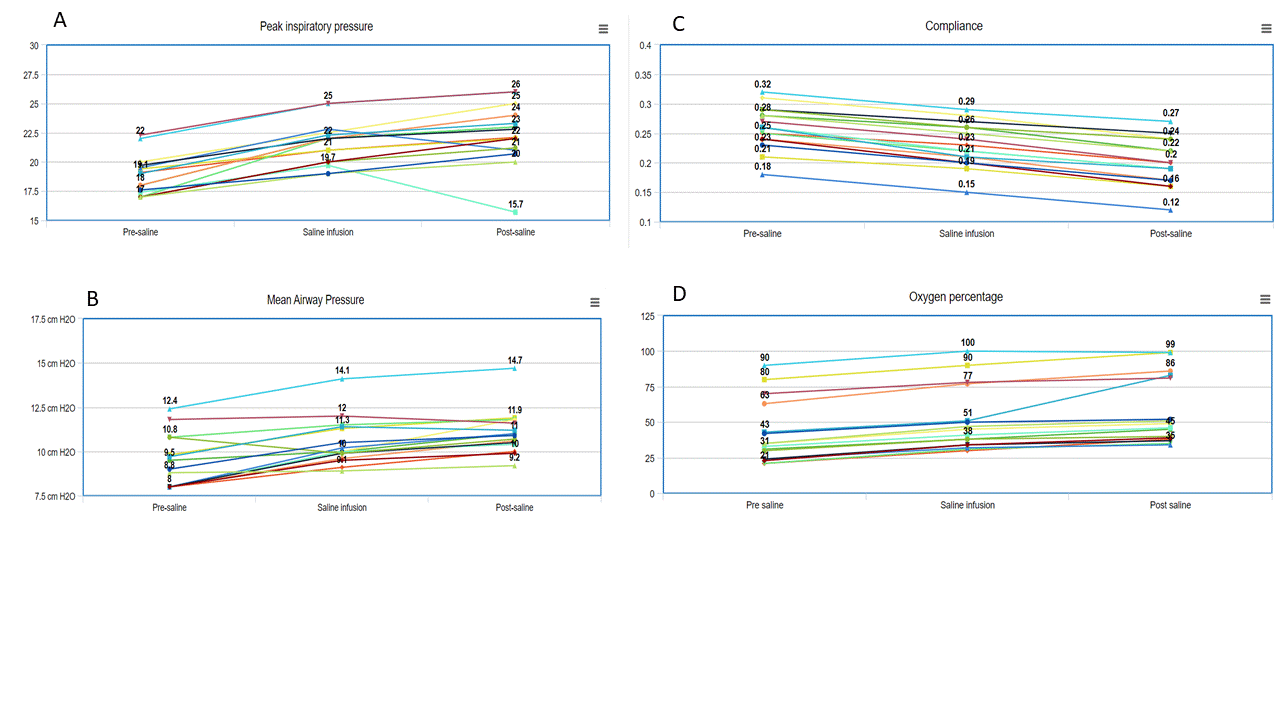
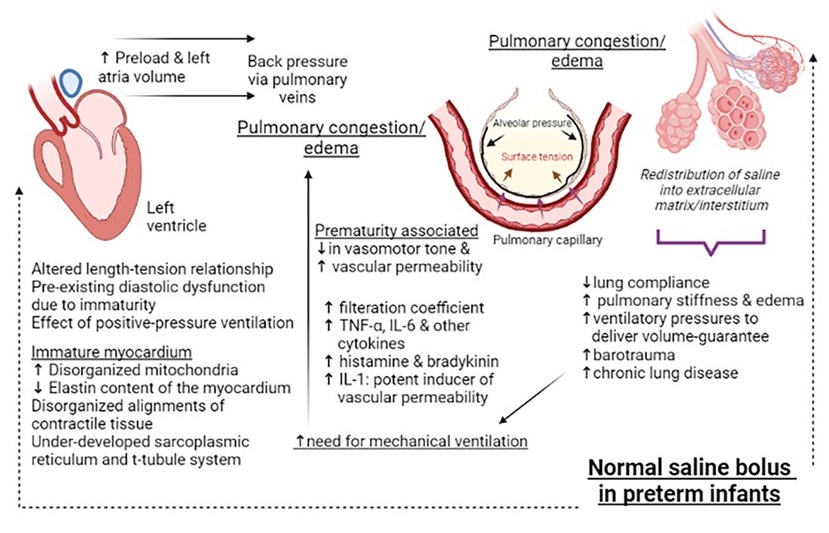
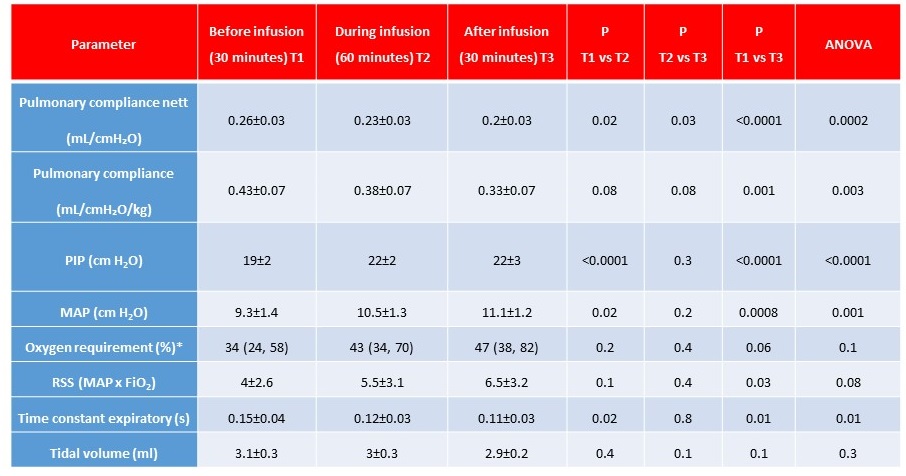
Results: Sixteen infants (mean GA 25.2±1 weeks and birthweight 620±60g) were administered normal saline (Table). None of the infants had an obvious cause of hypovolemia. The most common reason for saline bolus was hypotension (mean blood pressure [BP] < GA) (multiple reasons in some). No improvement in mean BP was noted (24±2 vs 26±3mm Hg, P=0.2). A significant reduction in pulmonary compliance was noted (Table). To be able to deliver the set tidal-volume via volume guarantee, the ventilator generated higher peak inspiratory pressures. An increase in respiratory severity score (mean airway pressure x FiO2) was noted. Figure depicts the trends in compliance, inspiratory pressures and oxygen requirements, before, during and after the saline infusion. Figure 2 depicts mechanistic links between saline infusion and changes in lung compliance.
Conclusion(s): Normal saline infusion therapy was associated with adverse pulmonary mechanics. Relevant pathophysiologic mechanisms include translocation of fluid across pulmonary capillaries affected by low vascular tone and heightened permeability in extremes of prematurity, back-pressure effects from raised left atrial volume due to immature left-ventricular myocardium; complemented by the effect of cytokine release from positive pressure ventilation.