Neonatology
Session: Neonatology Pulmonology Clinical Science 1: Bronchopulmonary Dysplasia
575 - Nitric Oxide Synthase Modulates the S-Nitrosoglutathione Reductase Inhibitor Rescue of Neonatal Oxygen-Induced Airway Hyperreactivity in Murine Precision-cut Lung Slices
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 575
Publication Number: 575.1354
Publication Number: 575.1354
- TR
Thomas M. Raffay, MD (he/him/his)
Associate Professor
Case Western Reserve University
Cleveland, Ohio, United States
Presenting Author(s)
Background: Bronchopulmonary dysplasia survivors display long-term obstructive lung disease with wheezing and airway hyperreactivity (AHR). We have previously shown in a mouse model that S-nitrosoglutathione (GSNO), an endogenous smooth muscle relaxant molecule produced by the activity of nitric oxide synthase (NOS), is degraded in neonatal hyperoxia by upregulated GSNO reductase (GSNOR) and in vivo oxygen-induced AHR can be rescued with a GSNOR inhibitor (Raffay, Mole Pharm 2016). This may have clinical implications in bronchopulmonary dysplasia as GSNO-based therapies are currently being evaluated in patients with asthma and cystic fibrosis.
Objective: We hypothesize that inhibition of GSNOR to rescue neonatal oxygen-induced AHR in precision-cut lung slice preparations requires NOS activity to produce endogenous GSNO.
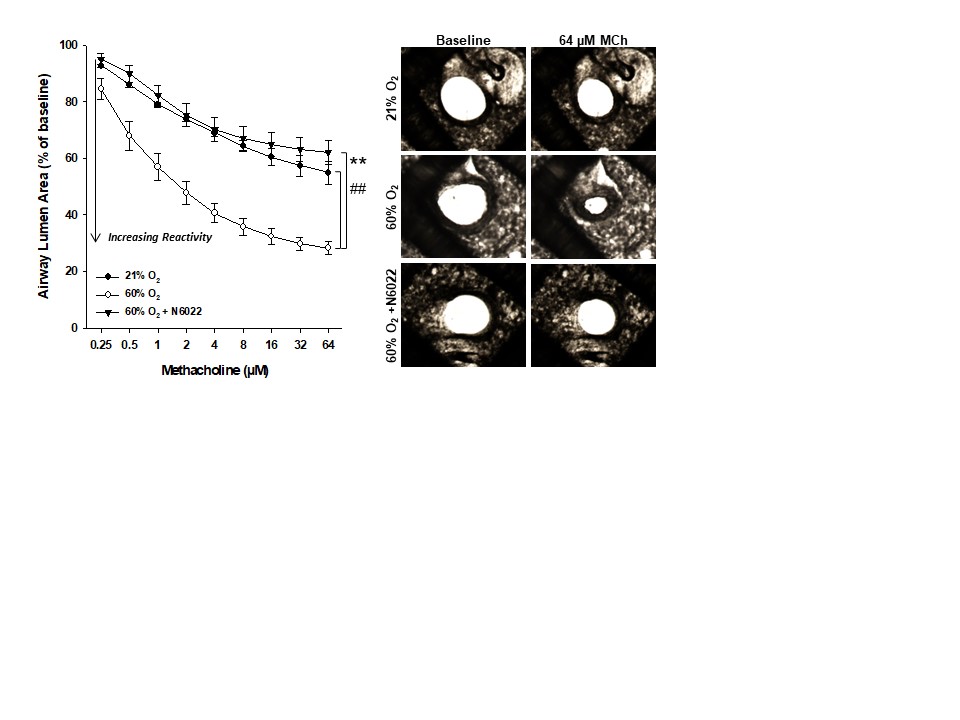
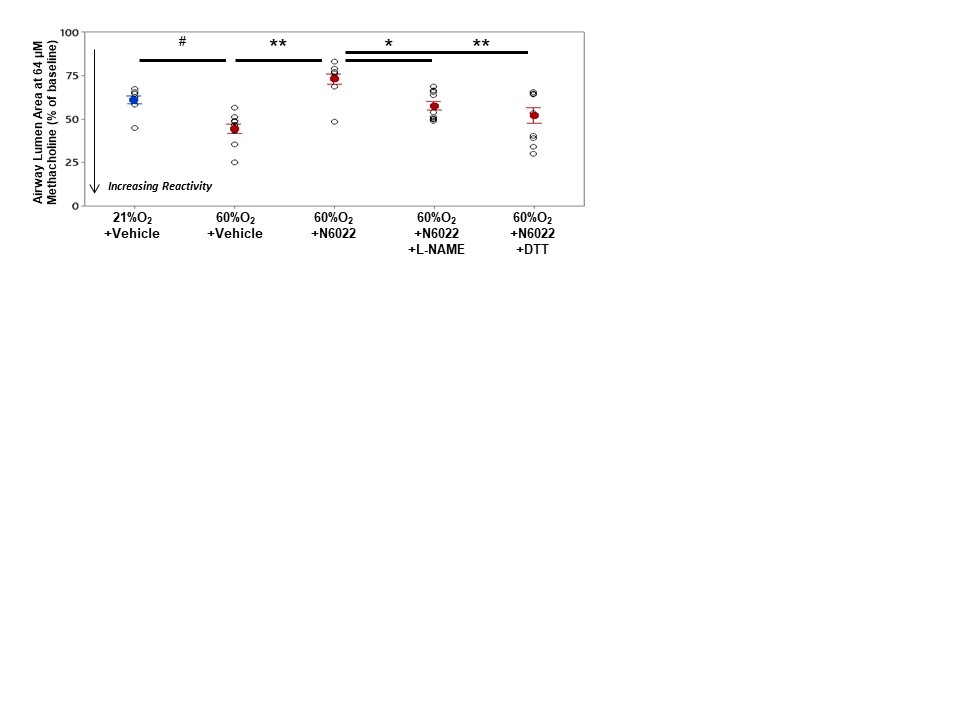
Design/Methods: Newborn C57BL/6 mice were randomized on the first day of life and assigned to room air (21% O2) or hyperoxic (60% O2) groups for three weeks to induce AHR. AHR was assessed in vitro using precision-cut living lung slice preparations in response to bath-applied methacholine (MCh) to cause airway constriction. Lung slices were pre-incubated overnight with or without a GSNOR inhibitor (N6022, 100μM) before generating MCh dose-response curves (0.25-64μM). Next in single-dose maximum concentration studies (MCh, 64μM), slices were also co-incubated for 30 min with the NOS inhibitor Nω-Nitro-L-arginine methyl ester (L-NAME, 100μM) or the thiol reducing agent dithiothreitol (DTT, 10μM). AHR is reported as the percent change in airway lumen area from baseline (Mean +/- 1 SEM). 2-3 airways were imaged and averaged per animal per condition.
Results: Neonatal hyperoxia significantly increased airway contractile responses to MCh and GSNOR inhibition attenuated AHR in the hyperoxic slices (Fig 1). Pre-incubation of hyperoxic slices with either L-NAME or DTT rendered the GSNOR inhibitor ineffective in rescuing airway responses to the maximal dose of MCh compared to vehicle (Fig 2).
Conclusion(s): These studies show that neonatal hyperoxia-exposed mice display in vitro AHR to MCh and that inhibition of GSNOR attenuates oxygen-induced AHR. Endogenous NOS activity to generate GSNO is likely required for this protective effect as the inhibition of NOS with L-NAME or the pretreatment with the thiol reducing agent DTT in the hyperoxic slices ablates the airway protection of GSNOR inhibition. These mechanisms will be important to further the investigation of GSNO-based therapies as novel treatments for wheezing disorders in bronchopulmonary dysplasia survivors.