Neonatology
Session: Neonatal Neurology 8: Preclinical
328 - Mechanistic Signatures of Synergistic Global and White Matter Neuroprotection by Azithromycin and Erythropoietin in the Preterm-Equivalent Ferret Brain
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 328
Publication Number: 328.2852
Publication Number: 328.2852

Thomas R. Wood, MD, PhD (he/him/his)
Assistant Professor
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: The ferret brain has many similarities with the human, particularly gyrification and white matter structure. We have shown that azithromycin (Az) and erythropoietin (Epo) are synergistically neuroprotective in ferret white matter, but the underlying biochemical and cellular responses are unexplored.
Objective: To examine transcriptomic and microglial signatures of combined Az and Epo neuroprotection in a postnatal day (P)14 extremely preterm-equivalent ferret ex vivo brain slice model of oxygen glucose deprivation (OGD).
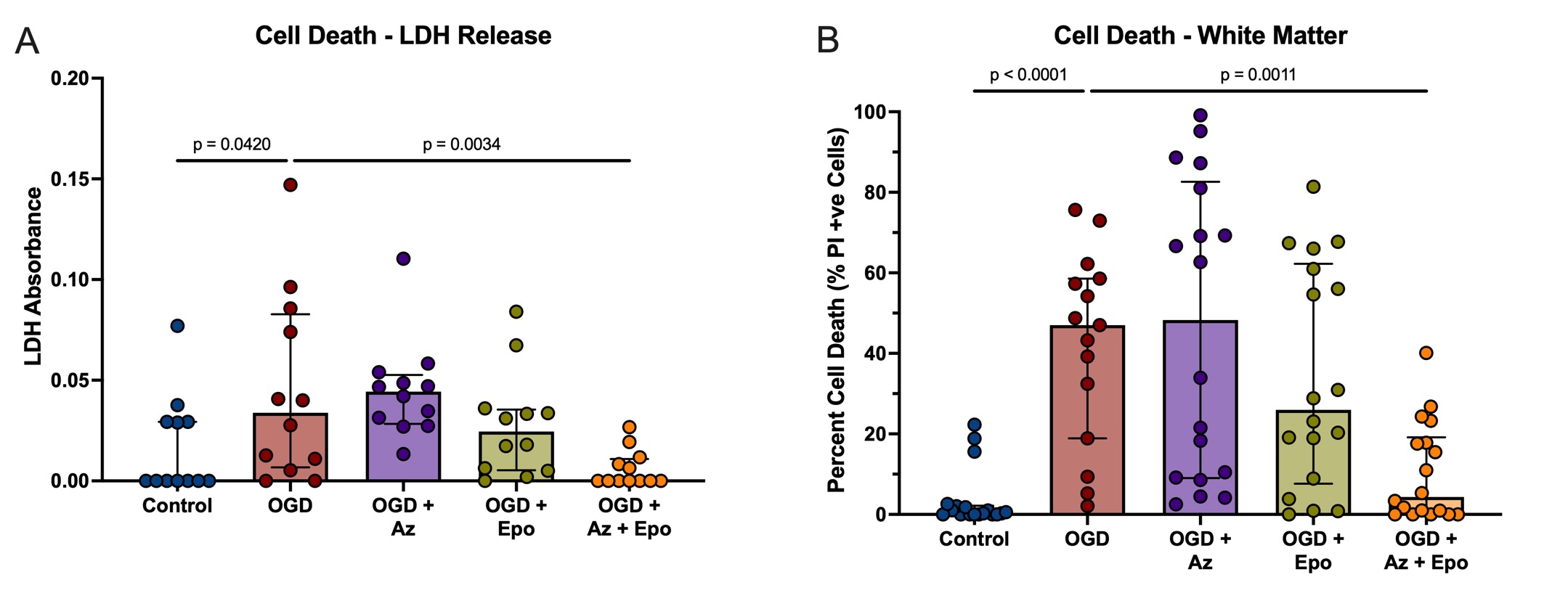
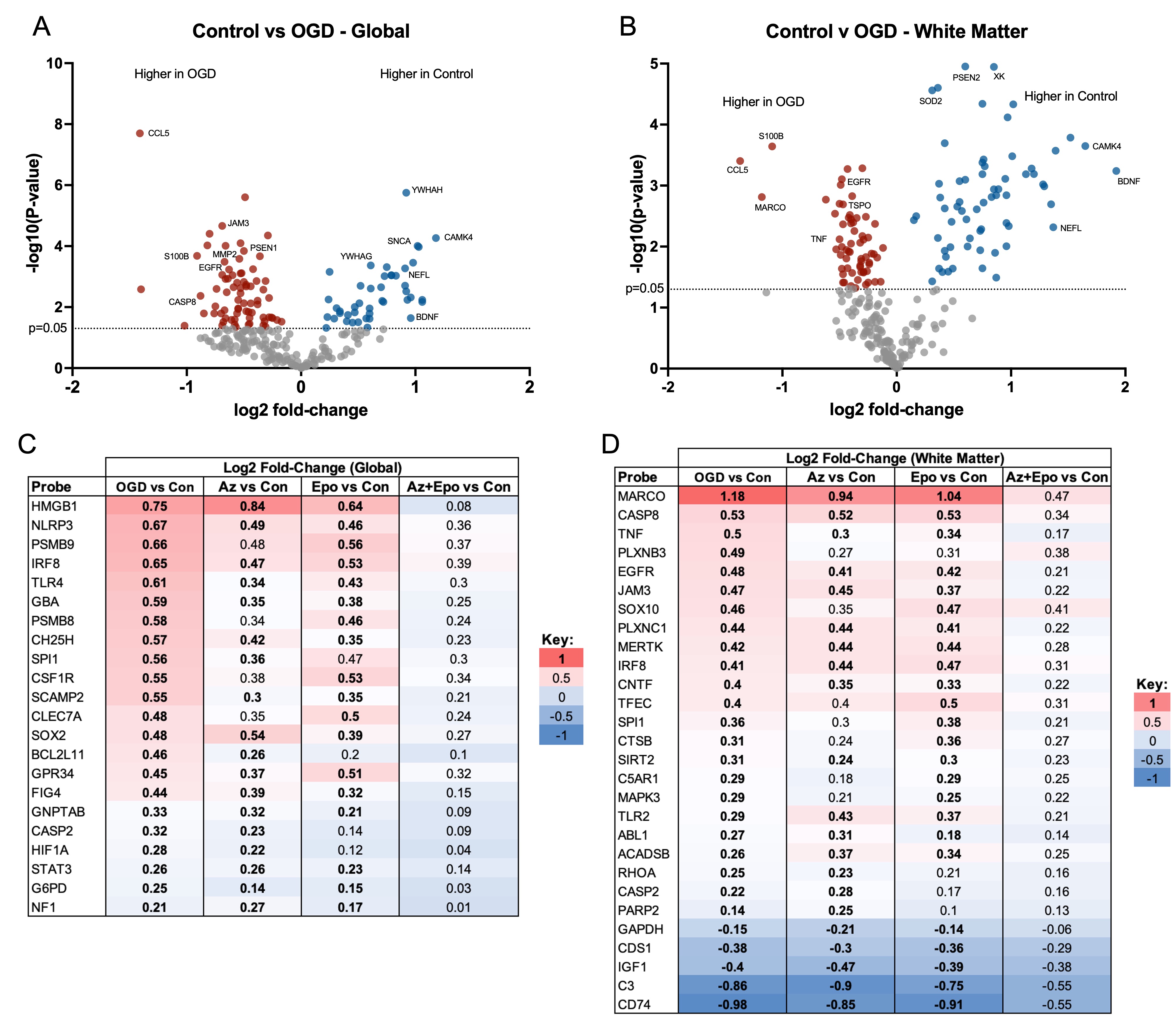
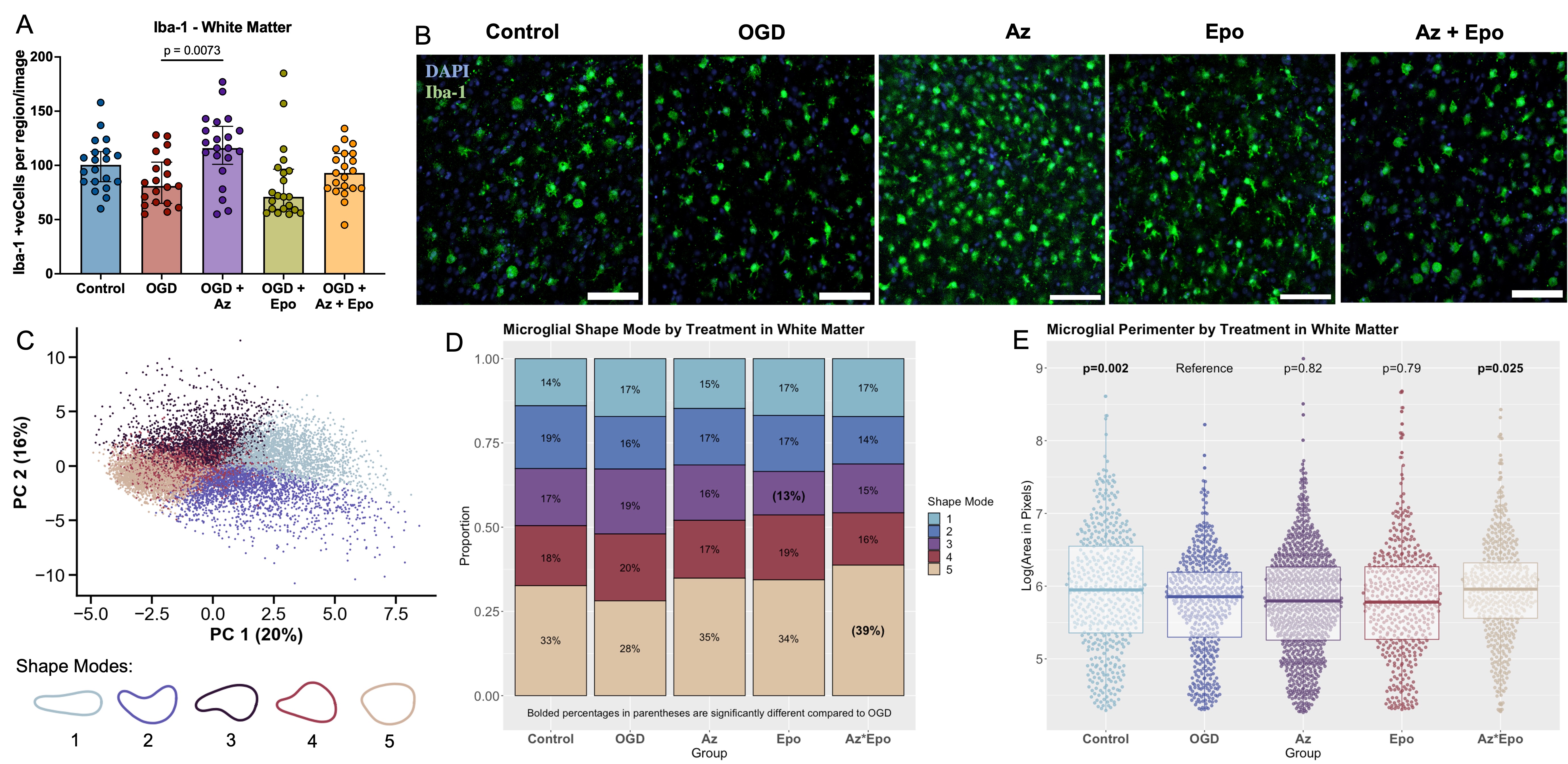
Design/Methods: 300μm whole-hemisphere live ferret brain slices were obtained at P14. After 72h rest, slices were subjected to 2h of OGD followed by treatment with Epo (3 IU/mL), Az (15uM), or Az+Epo for 24h. Global and regional cell death were determined using lactate dehydrogenase (LDH) release and confocal microscopy of propidium iodide (PI)-stained cells, respectively. Global (whole slice) and regional white matter transcriptomics were analyzed using a ferret-specific NanoString nCounter panel of 255 genes. Transcripts upregulated by OGD and normalized by Az+Epo were determined. Microglial morphology was assessed using a machine learning pipeline to calculate morphological parameters as well as five morphological groupings (shape modes).
Results: Global and white matter cell death were significantly reduced by Az+Epo but not either drug individually (Fig 1). After OGD, n=117 (global) and n=129 (white matter) genes were differentially expressed (Fig 2A-B). Of these, n=22 (global) and n=28 (white matter) were normalized to healthy control levels by Az+Epo but not Az or Epo individually. The top transcripts normalized by Az+Epo were HMGB1 and NLRP3 globally (Fig 2C), and the macrophage scavenger receptor MARCO, Caspase 8, and TNF in white matter (Fig 2D). In the white matter, Az, but not Epo or Az+Epo resulted in increased microglial numbers (Fig 3A-B). Synergistic neuroprotection by Az+Epo in the white matter was associated with an increase in the number of one specific microglial shape mode (Fig 3C-D), as well as normalization of microglial perimeter – a feature that indirectly captures cell branching – after OGD (Fig 3E).
Conclusion(s): In the extremely preterm-equivalent ferret brain, Az+Epo provide synergistic neuroprotection globally and in white matter via mitigation of cell death pathways (HMGB1, Caspase 8), inflammatory responses (NRLP3 inflammasome, MARCO, TNF), and morphological microglial changes that are not seen with either drug individually. These pathways warrant further investigation as targets for regional and global neuroprotection in the extremely preterm brain.