Neonatology
Session: Neonatal Pulmonology - Clinical Science 3: Non-Invasive Respiratory Support, RDS
223 - Early (≤2hour) bolus surfactant replacement therapy versus standard care in term (≥37 weeks) neonates with meconium aspiration syndrome: an open label randomized control trial
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 223
Publication Number: 223.2757
Publication Number: 223.2757
- SN
Sushma Nangia, MBBS, MD, DM
Professor
Lady Hardinge Medical College & Kalawati Saran Children's Hospital
New Delhi, Delhi, India
Presenting Author(s)
Background: Meconium aspiration syndrome (MAS), a common cause of respiratory failure in late preterm and term neonates, is associated with high mortality and morbidity. Amongst all the treatment modalities available for severe MAS, surfactant administration has got a proven role in decreasing the incidence of progressive respiratory failure as evidenced by the significant reduction in mortality or need for extracorporeal membrane oxygenation
Objective: To evaluate the effect of early bolus surfactant therapy as compared to standard care on total duration of respiratory support in term neonates (≥37weeks) with meconium aspiration syndrome
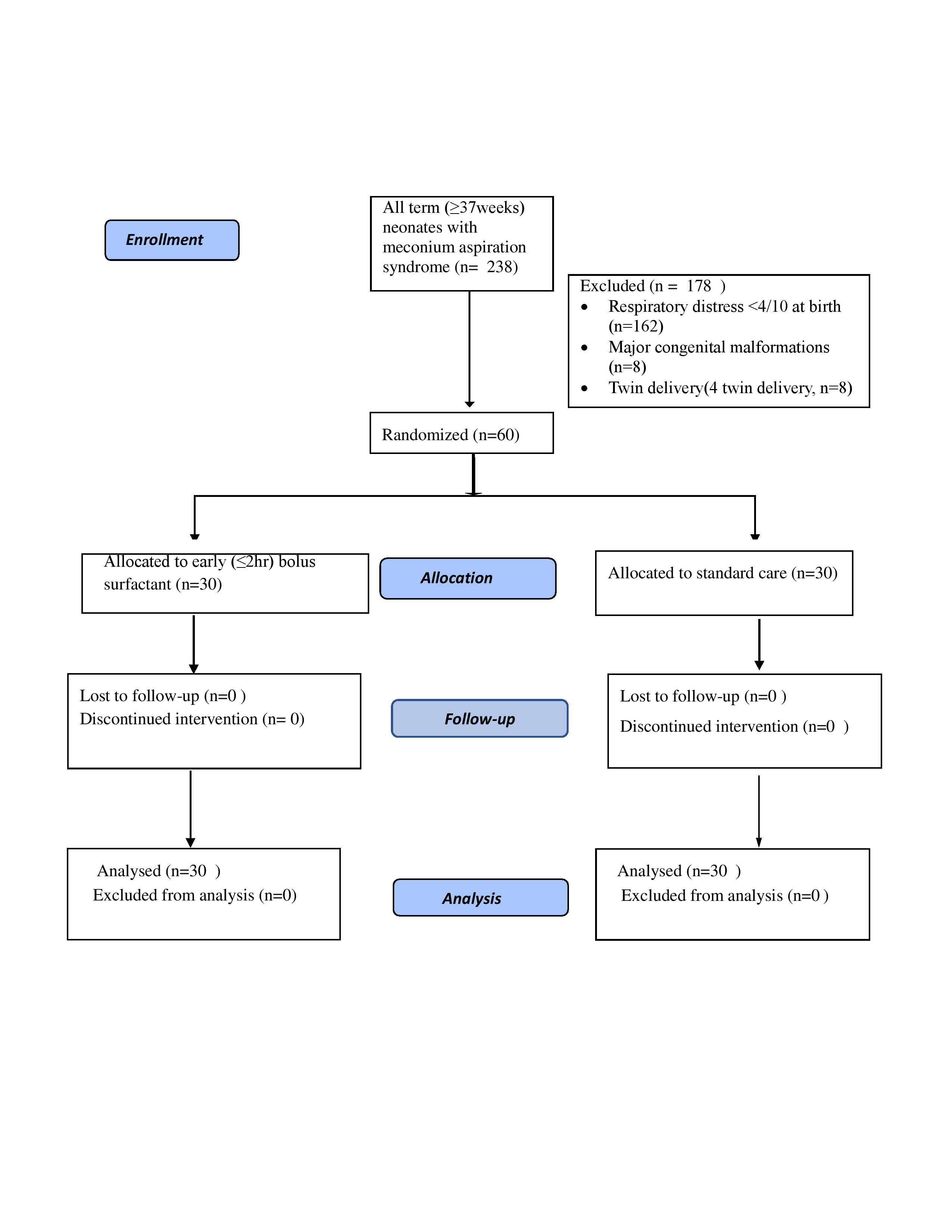
Design/Methods: This randomized controlled trial enrolled term neonates (≥37 weeks) with meconium aspiration syndrome. Neonates randomized to 'surfactant group' (n=30) received bolus surfactant replacement treatment within two hours after birth. However, those randomized to ''standard care group” (n=30) were managed as per the current standard protocol. No placebo was used in the standard care group. The primary outcome variable was total duration of respiratory support (invasive and non-invasive). Secondary outcome variables were severity of MAS, development of hypoxic ischemic encephalopathy (HIE), complications associated with MAS (air leaks, PPHN, sepsis), duration of hospitalization, and mortality during hospital stay.
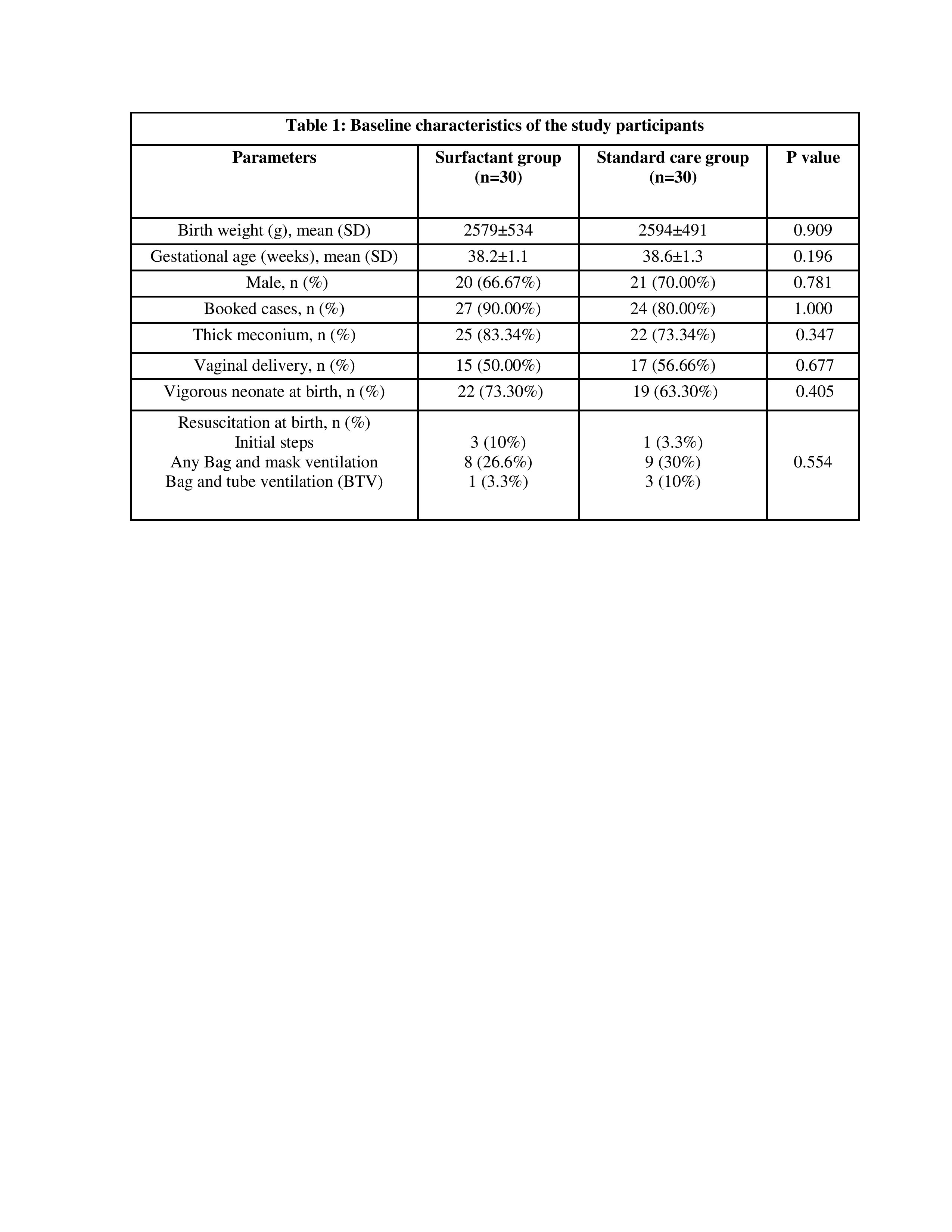
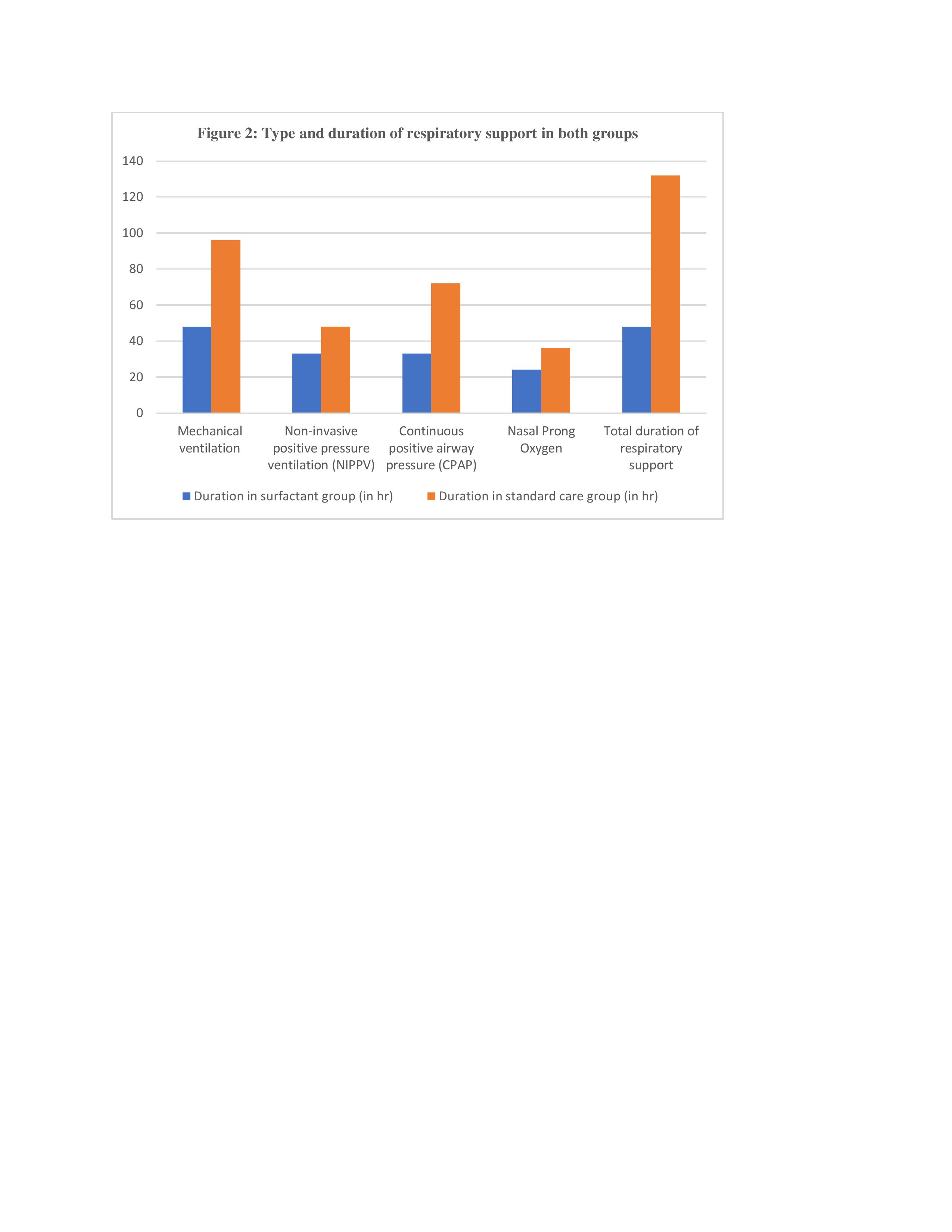
Results: Baseline characters including birth weight and gestational age were similar between the two groups. The median (IQR) total duration of respiratory support was significantly lower in the surfactant group as compared to the standard care group (48 [24–120] h vs 132 [72–216] h; p 0.003). Among secondary outcome variables moderate to severe MAS was present in 6 (20%) vs. 12 (40%) neonates in ‘surfactant group’ and ‘standard care group’ respectively (OR 0.16 (0.052-0.52); p=0.002). Other parameters like incidence of HIE, complications associated with MAS (air leaks, PPHN, sepsis), duration of hospitalization, and mortality during hospital stay were comparable
Conclusion(s): Early bolus surfactant replacement therapy within 2 hours of life significantly reduces the total duration of respiratory support among term neonates (≥37weeks) with MAS without increasing adverse outcomes.