Neonatology
Session: Neonatal Fetal Nutrition & Metabolism 1: Growth of Body and Brain
118 - Intranasal Insulin Treatment Partially Recovers Synaptic Gene Expression and Energy Metabolism in the Brain Regions of Formerly Iron Deficient Adult Rats
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 118
Publication Number: 118.498
Publication Number: 118.498
- KE
Kathleen M. Ennis-Czerniak, BA
Researcher
University of Minnesota
Woodbury, Minnesota, United States
Presenting Author(s)
Background: Perinatal iron deficiency (PID) impairs energy metabolism, myelination, and synaptogenesis in the developing hippocampus (HPC) and cortex (CTX) that persist into adulthood despite ID repletion. Intranasal insulin (INS) improves HPC-mediated memory function in adult humans with cognitive deficits. In developing rats, INS during iron treatment partially normalizes the HPC transcriptome altered by PID. It is unknown if INS treatment in adulthood after brain ID correction will ameliorate altered mitochondrial bioenergetics, synaptogenesis and myelination in the brain regions of adult rats with PID.
Objective: Examine whether INS ameliorates altered synaptogenesis, myelination and mitochondrial bioenergetics in the brain regions of formerly ID (FID) adult rats.
Design/Methods: PID was induced using a low iron diet from gestational day 3 through postnatal day (P) 7, followed by an iron sufficient (IS) diet until adulthood (P90; FID group). IS group received IS diet throughout. INS was administered to the IS-INS and FID-INS groups twice daily from P90-P104. mRNA expression of iron transporter (Tfrc), markers of synaptogenesis (Camk2a, Psd95, Nr2b), and myelination (Mbp) was determined using qPCR and compared with IS and FID groups treated with intranasal saline (IS-CON and FID-CON) (N=4-5/group). Oxygen consumption rate (OCR) was quantified in mitochondria isolated from the CTX.
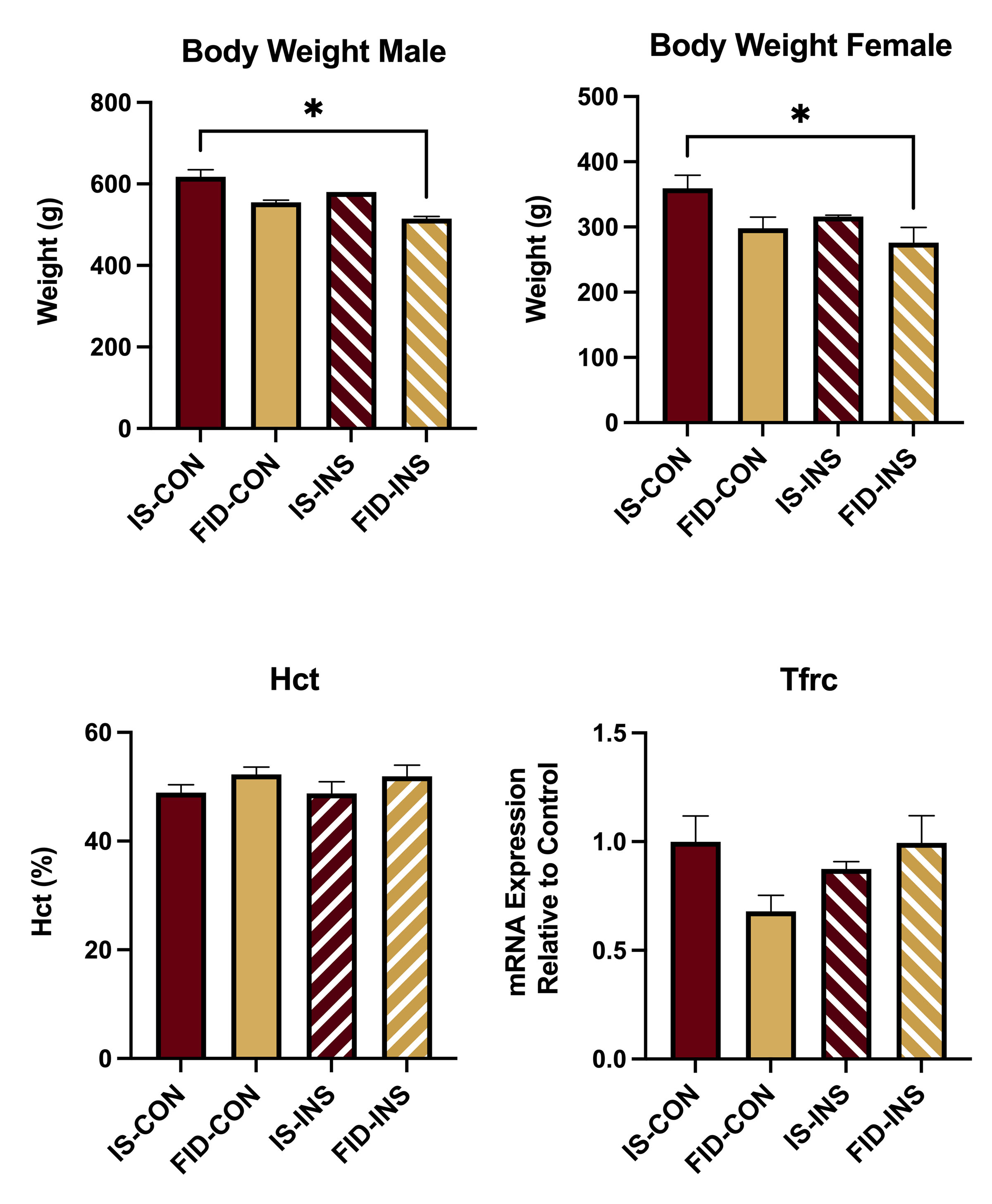
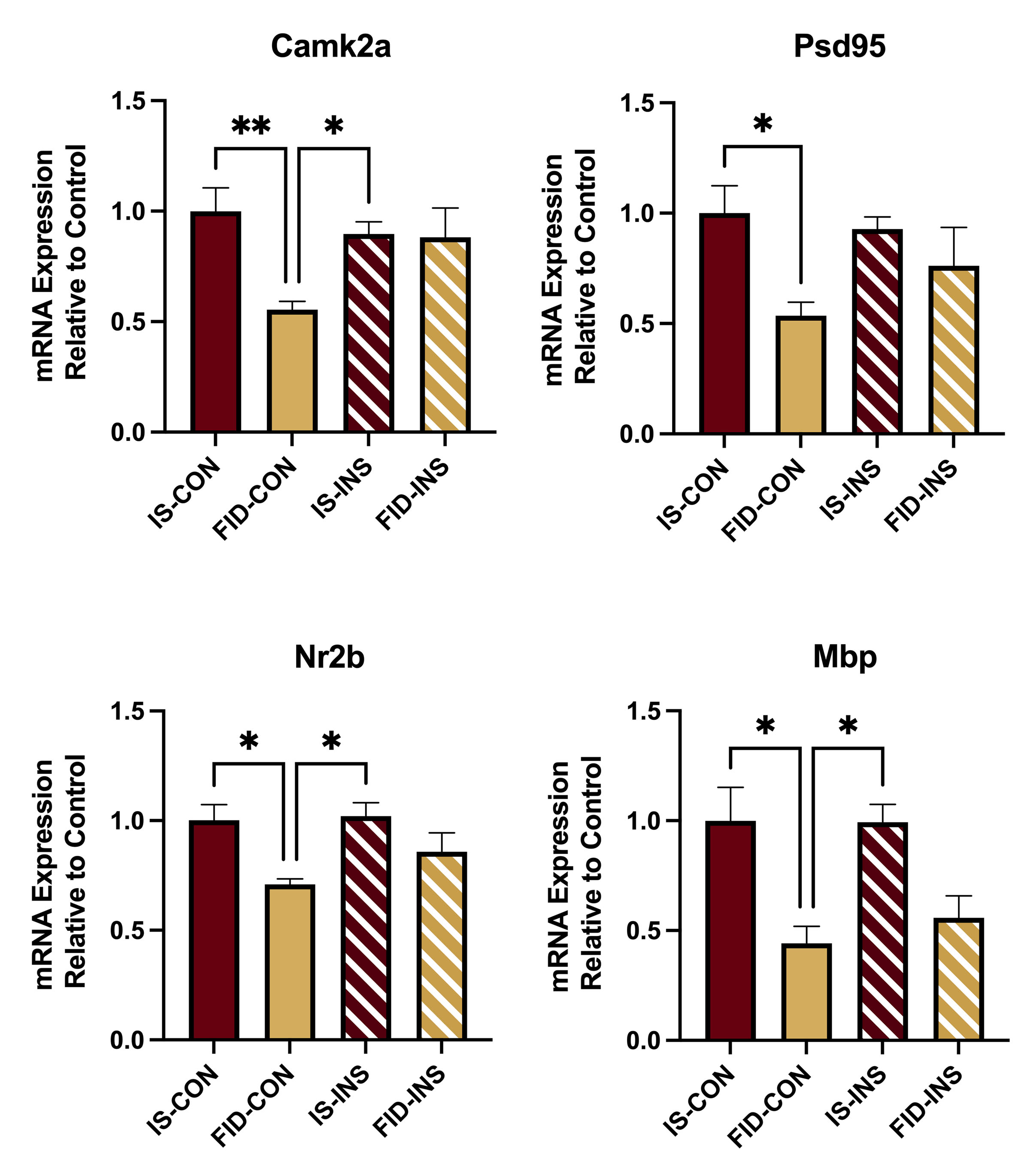
Results: Body weight was lower in the FID-INS group, compared with the IS-CON group (Fig 1). Hct and Tfrc mRNA expression were not different among the groups (Fig. 1). Camk2a, Psd95, Nr2b and Mbp mRNA expression was decreased in the FID-CON group compared to the IS-CON and IS-INS groups (Fig 2). There was a trend towards normalization of all transcripts, except Mbp, in the FID-INS group (Fig 2). Basal mitochondrial OCR was reduced in the FID group and partially recovered in the FID-INS group (Fig 3).
Conclusion(s): Comparable Hct and Tfrc expression among the groups suggest repletion of HPC iron in the FID groups. Decreased Camk2a, Psd95, Nr2b and MBP in the FID-CON group suggest persistently decreased synaptogenesis, and myelination in the HPC. A trend towards normalization of Camk2a, Psd95 and Nr2b in the FID-INS group suggest partial recovery of synaptogenesis-associated transcripts with INS treatment in adulthood. Lack of a similar change in Mbp expression suggests adverse effect of PID on myelination is recalcitrant to INS treatment. Improved OCR in the CTX of INS treated FID animals suggests improved mitochondrial bioenergetics with INS. INS may be considered as an adjunct therapy for overcoming the adverse effects of perinatal ID.