Neonatology
Session: Neonatal Neurology 8: Preclinical
330 - Novel Autoantibody Discovery with Programmable Phage Display in Autism Spectrum Disorders
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 330
Publication Number: 330.3097
Publication Number: 330.3097

Melody Lun, MD PhD (she/her/hers)
Clinical fellow
University of California, San Francisco, School of Medicine
San Francisco, California, United States
Presenting Author(s)
Background: Autism spectrum disorder (ASD) is a heterogeneous syndrome with hallmark features of social deficits and repetitive behaviors. The prevalence of ASD has risen dramatically, estimated to affect 1 in 54 children in the US. While there has been tremendous effort to define the genetic etiologies of ASD, these account for only 40% of cases. Autoimmunity is suspected to be an additional risk factor, yet few antigenic targets have been identified thus far due to challenges in profiling autoimmune reactivities.
Objective: The goal of this study is to define the autoimmune repertoire in cerebrospinal fluid (CSF) of children with ASD and to generate candidate autoantibodies for in vivo modeling of autoantibody pathogenicity.
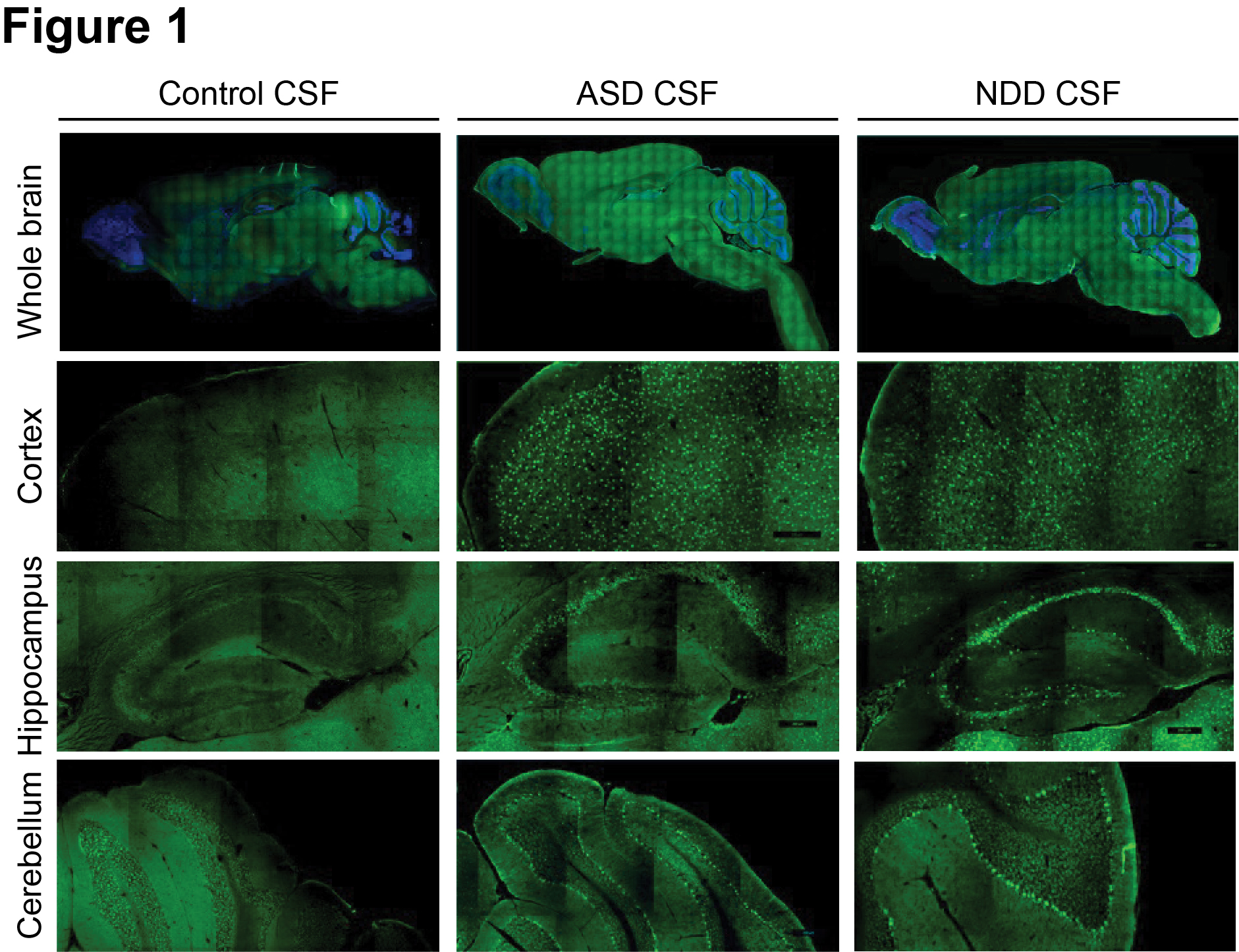
Design/Methods: We evaluated CSF from children with ASD (n=36), other neurodevelopmental disorders (NDD, n=20), and surgical controls (n=34). By immunofluorescence on mouse brain tissues, we determined the presence of anti-brain reactivities in CSF and defined their anatomic and subcellular distributions. Next, we performed phage display immunoprecipitation sequencing (PhIP-Seq) with a human peptidome library to identify antigenic targets. Candidates were validated by multiple approaches, including luciferase-based and HEK293T cell-based assays.
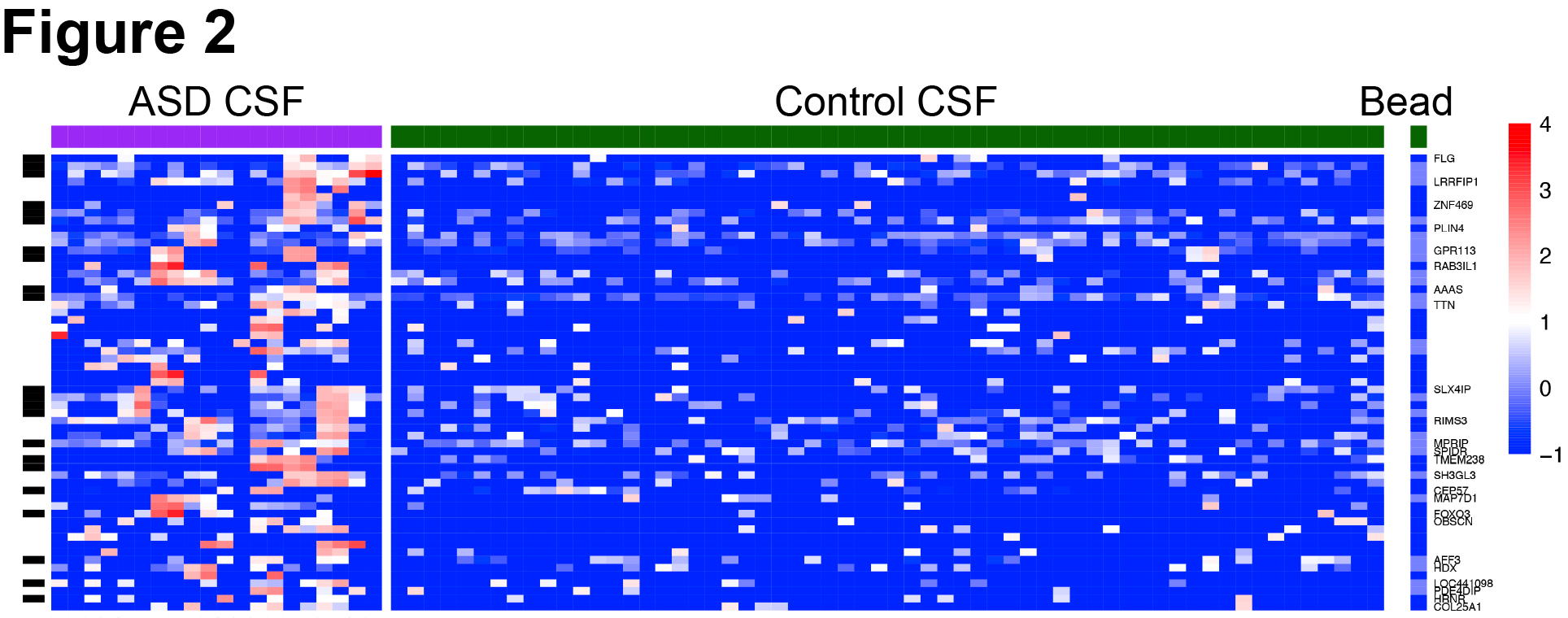
Results: CSF from patients demonstrated robust immunostaining in the mouse cortex, hippocampus, and cerebellum. There was higher prevalence of anti-brain reactivity in CSF from children with ASD (10 of 36, 27.8%) and NDD (6 of 20, 30%) compared to surgical controls (4 of 34, 11.7%). By PhIP-Seq, we profiled the autoantibody repertoires and limited our analysis to 10 ASD cases with positive anti-brain staining versus 30 control cases with negative anti-brain staining. This analysis yielded 24 differentially enriched peptides. By luciferase-based assay, we validated presence of anti-FOXO3 autoantibody in an ASD patient (A013) with history of ganglioglioma. We performed additional epitope mapping to confirm antibody binding within the transactivation domain of FOXO3. By HEK293T overexpression cell-based assays, A013 CSF antibodies colocalized with anti-FOXO3 commercial antibody immunostaining and Western blot signals.
Conclusion(s): This study is the first to comprehensively profile CSF from a large cohort of children with ASD and NDD. While this study did not identify a uniform autoantibody signature across this cohort, we report a novel autoantibody against FOXO3, a transcription factor that is a key regulator of neural stem cell maintenance and neuronal plasticity. Additional studies are needed to determine the role of anti-FOXO3 in ASD pathogenesis.
.jpg)
