Neonatology
Session: Neonatal Neurology 9: Preclinical
345 - Spatial Cell-Specific Transcriptomic and Proteomic Analyses of Genes Related to Cognitive Decline in a Ferret Model of Preterm Brain Injury
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 345
Publication Number: 345.2860
Publication Number: 345.2860

Olivia C. Brandon, BS (she/her/hers)
Research Scientist
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: Adults born preterm have higher rates of mortality and cognitive decline, including dementia and Alzheimer’s disease (AD), but it is unclear how these processes might be best studied in animal models. In the general population, females are also at a higher risk of AD compared to males. The ferret provides a model for the human brain due to several shared physical and developmental attributes.
Objective: To investigate aging-related regional and cell-specific transcriptomic and proteomic responses of oligodendrocytes (Olig2) and microglia (Iba-1) in ferret brains after a preterm-equivalent inflammation-sensitized hypoxic-ischemic injury.
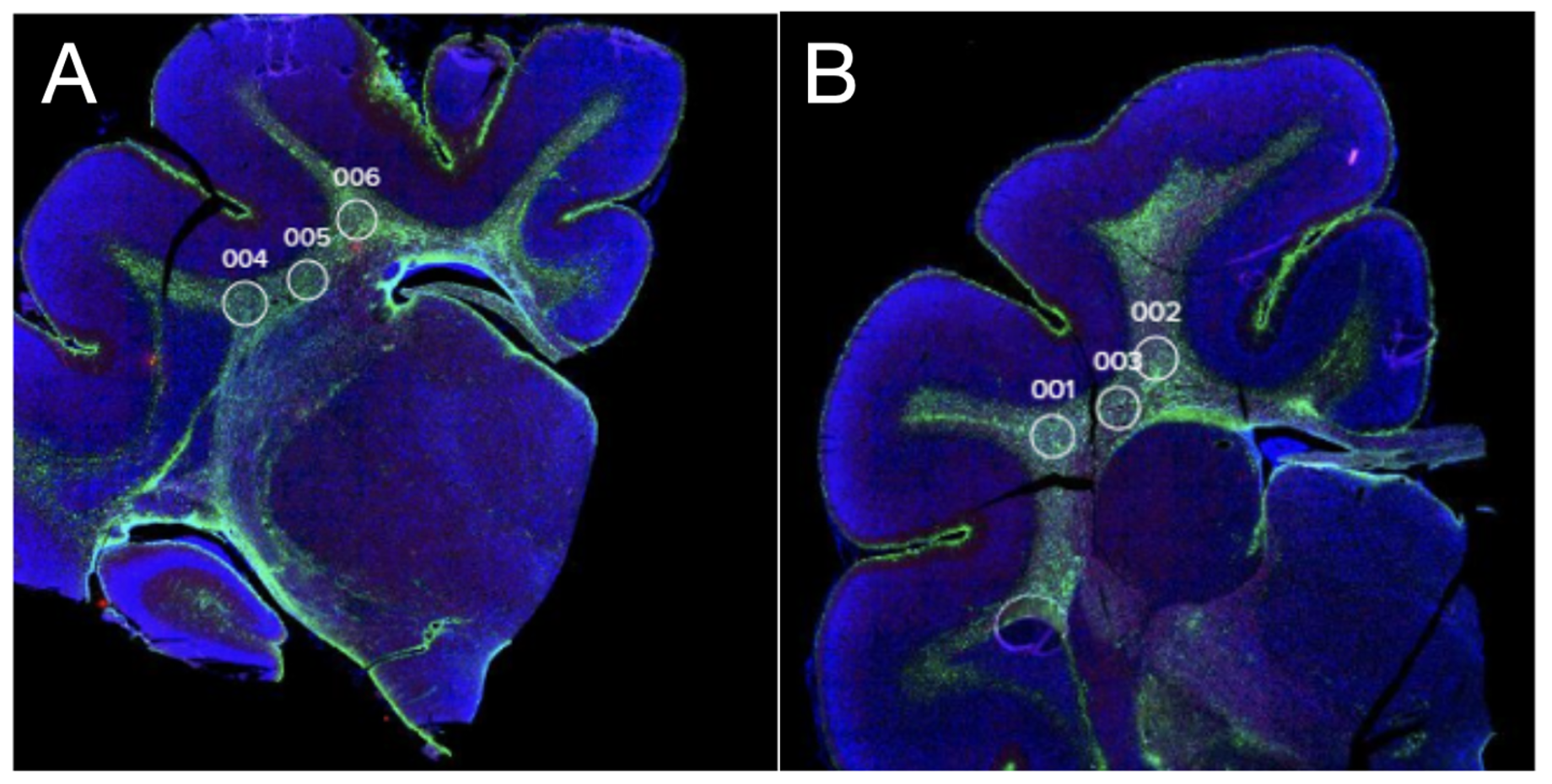
Design/Methods: We used the NanoString GeoMx spatial transcriptomics platform (18,676 human genes, ~80% of which have suitable sequence homology with the ferret) in brains from two female postnatal day (P) 42 ferret brains. On P17, equivalent to 32-36 weeks’ gestation, the injured ferret received Lipopolysaccharide (LPS) administration and bilateral ischemia followed by hypoxia-hyperoxia-hypoxia, modelling preterm brain injury. The other brain was a littermate control. Tissue was collected on P42 (childhood-equivalent), and sections taken from formalin-fixed- paraffin-embedded blocks. Regions of interests were focused on white matter, which is particularly vulnerable to preterm brain injury and is also critical to executive function in dementia (Figure 1). Gene/protein levels from Olig2 and Iba-1 positive cells (3 regions per animal per cell type) were determined and compared.
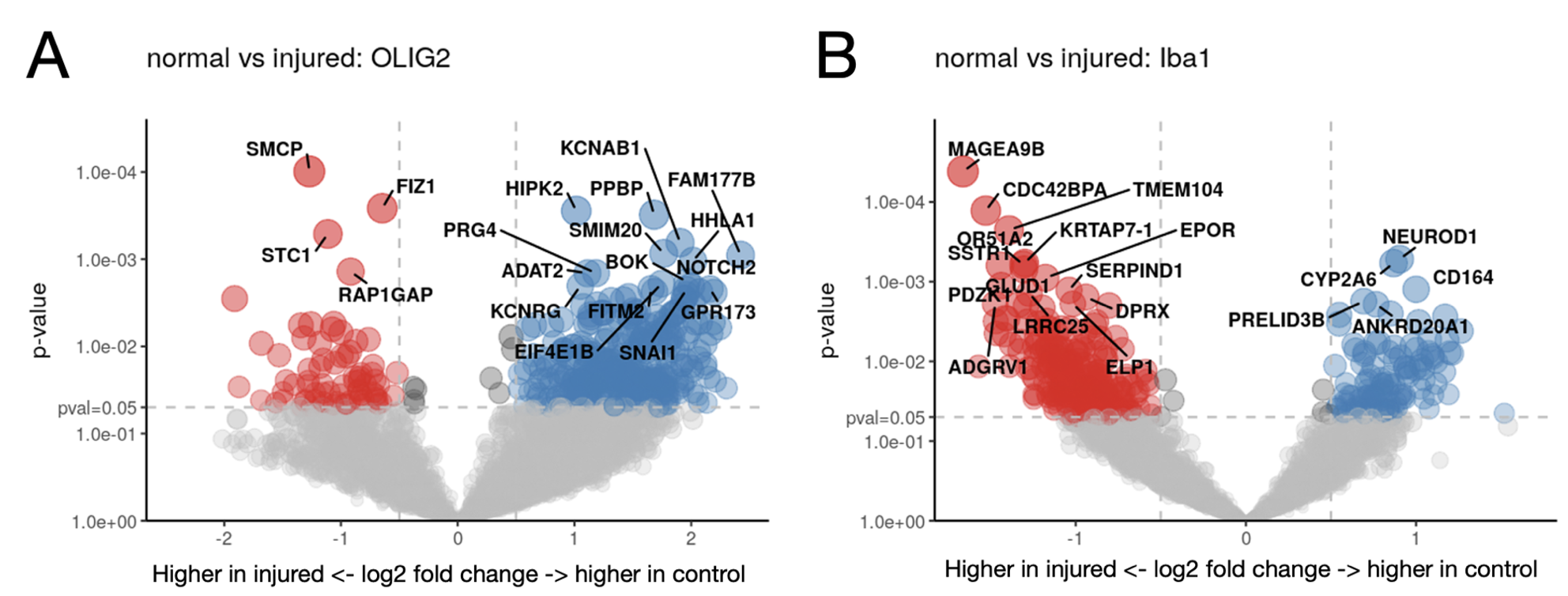
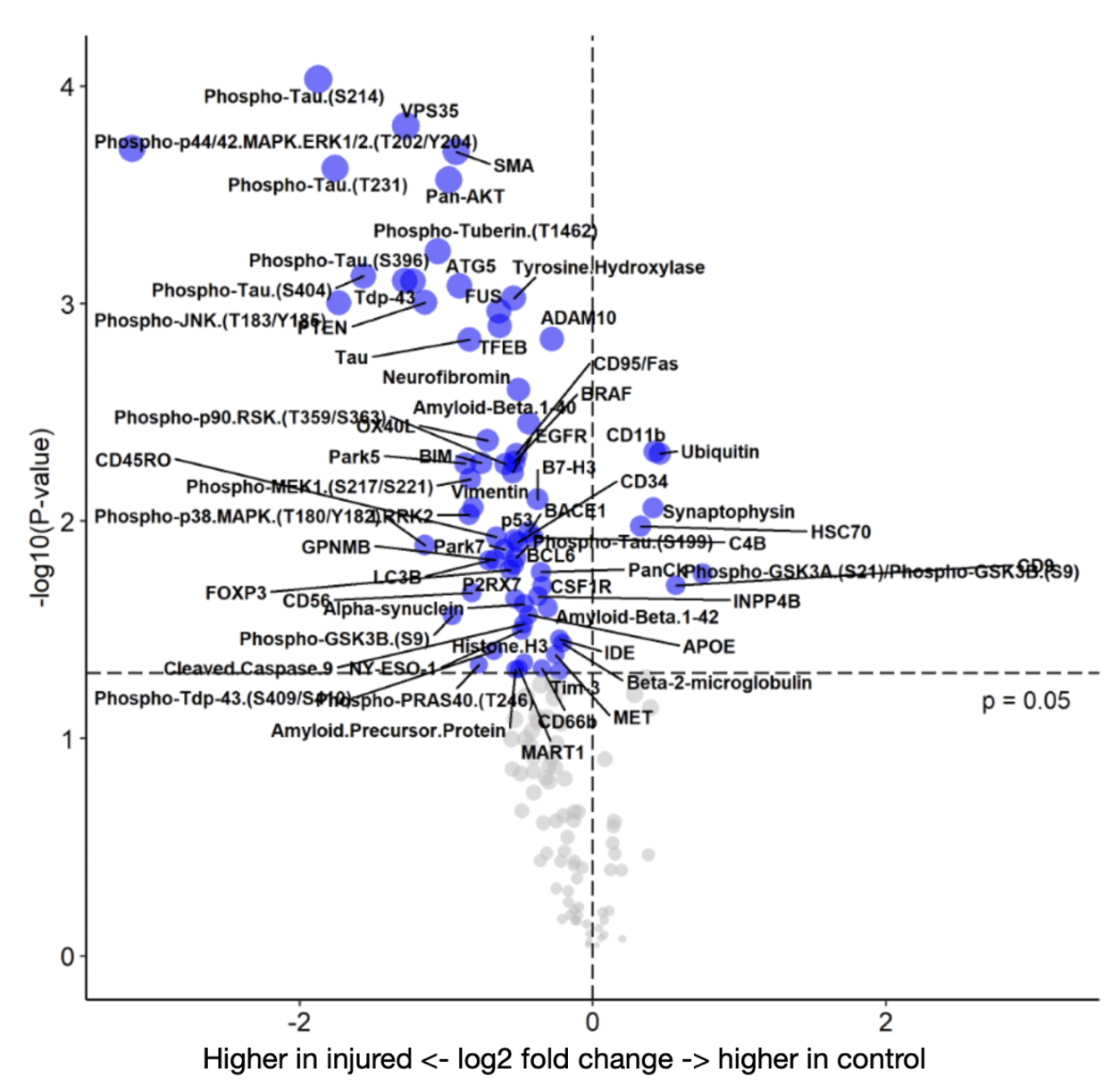
Results: In Olig2 positive cells, the injured brain had significantly increased gene expression for SMCP and FIZ1, genes relating to mitochondria and zinc ion regulation, while the control brain showed increased expression of PPBP and HIPK2, relating to cytokines, platelet activation, and cell growth (Figure 2A). In control Iba-1 positive cells, NEUROD1, a transcription factor involved with the differentiation of neural cells, was upregulated (Figure 2B). Several key proteins implicated in cognitive decline and AD were upregulated in the injured brain including phospho-tau, Tau, Phospho-JNK, amyloid precursor protein (APP), Histone H3, and amyloid-beta (Figure 3).
Conclusion(s): Preterm birth is a risk factor for the cognitive decline, dementia, and AD. In this late preterm model of brain injury in the ferret, proteins associated with AD such as phospho-tau, alpha synuclein, APP, and amyloid-beta were upregulated several weeks after brain injury. Additional studies are needed to investigate whether this model is suitable for exploring mechanisms of preterm-related brain pathology in later life.