Neonatology
Session: Neonatal Cardiology and Pulmonary Hypertension 1: PDA
88 - Investigation of a Functional rs66698963 Insertion-Deletion Polymorphism That May Contribute to Patent Ductus Arteriosus (PDA)
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 88
Publication Number: 88.2184
Publication Number: 88.2184

Kristy Herrman, MD
Assistant Professor
University of Texas at Austin Dell Medical School
Austin, Texas, United States
Presenting Author(s)
Background: PDA is a common complication of prematurity and is associated with adverse outcomes. Closure of the PDA is inhibited by prostaglandin E2 (PGE2) infusion or intrinsic production via the arachidonic acid pathway.
Arachidonic acid (ARA) is converted to more than 100 signaling eicosanoids such as prostaglandins, leukotrienes, and thromboxanes. The final step in ARA synthesis is mediated by the enzyme FADS1 (fatty acid desaturase 1).
Although numerous studies demonstrate ARA levels are associated with genotype in the region of FADS1, one functional polymorphism has been associated with FADS1 mRNA and with circulating ARA levels in humans. The insertion-deletion (Indel) polymorphism rs66698963 is located in intron1 of FADS2 just downstream of FADS1. Individuals homozygous for the I allele (II) have been shown to have circulating ARA 50% higher than those homozygous for the D allele (DD), with heterozygotes (ID) intermediate, in 1504 healthy Chinese adults. Measurements in adults from the United States, United Kingdom and India have shown consistent patterns premature infants have not been studied.
Objective: To investigate if premature infants with FADS2 II genotype have greater ARA levels, more significant PDAs, and more need for catheter-based closure than DD genotype.
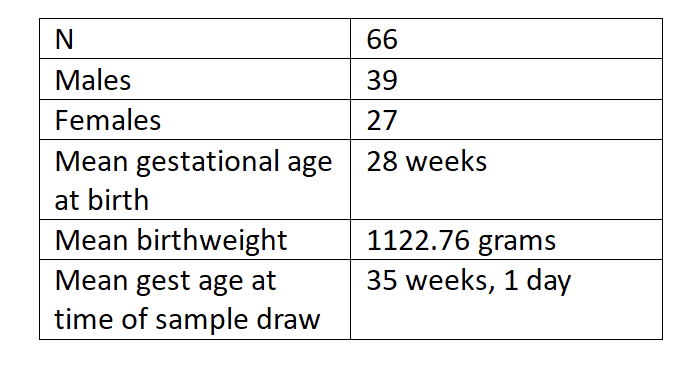
Design/Methods: This was a prospective observational study. All IRB and institutional approvals were obtained before informed consent was taken from parents of eligible newborns. The mean gestational age was 28 weeks. Blood was taken by heel prick and fatty acids quantitatively analyzed by gas chromatography. Indel status was determined by PCR and agarose gel electrophoresis. ANOVA was performed to assess significance between the three genotypes (I/I, I/D and D/D). A Tukey HSD post hoc test was conducted to further examine the significance between mean values of paired genotypes. P < 0.05 was considered significant.
Results: The rs66698963 Indel genotype and fatty acid profiles were available for 66 premature infants. In this sampling, D/D genotype was 41% (n = 27), I/D 47% (n = 31), and I/I 12% (n = 8). The ratio of ARA to precursor DGLA increases by 25% from DD to II genotype and is statistically significant (p=0.025). Among the 66 genotyped individuals 6 had Piccolo occlusion for PDA closure. Four of those are II and ID genotype carriers, two DD genotype.
Conclusion(s): This is the first study in premature infants to show that the insertion allele enhances pro-inflammatory ARA synthesis. Larger studies are warranted to determine any impact on the persistence, severity, or need for catheter -based closure of a PDA.