Neonatology
Session: Neonatal Neurology 4: Clinical
521 - Therapeutic opioid exposure for premature infants in the NICU is associated with altered thalamic connectivity at term
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 521
Publication Number: 521.1686
Publication Number: 521.1686
.jpg)
Kevin M. Cook, PhD (he/him/his)
Assistant Professor
Children's National Health System
Washington, District of Columbia, United States
Presenting Author(s)
Background: Preterm birth accounts for 10% of births worldwide, with earlier born infants typically experiencing lengthy NICU stays. Often these vulnerable infants receive opioid medications to manage pain and hyperalgesia, although some limited studies suggests that preterm opioid exposure is associated with differences in brain morphometry as well as later pain hypersensitivity and motor delays. However, it’s unclear how opioid exposure alters functional brain development during the third trimester, a critical period for the formation of early brain networks.
Objective: To determine the impact of opioid pain interventions during the preterm period on functional connectivity of the thalamus, the primary somatosensory relay hub, at term equivalent age (TEA).
Design/Methods: We prospectively recruit preterm infants born before 32 weeks postmenstrual age. We collected opioid information on all infants, including morphine and fentanyl. All opioid dosages were converted to morphine equivalency in mg/kg and then summed to measure cumulative opioid exposure (COE). Total number of painful procedures, including heel lances, IV placements, and venipunctures, were collected to include as a covariate of pain exposure. At TEA (mean=39.9 wks), infants underwent a resting state fMRI scan. Following preprocessing, right and left thalamic connectivity maps were calculated for each infant. Group-level regressions were then performed to assess the relationship between connectivity and COE, controlling for pain exposures.
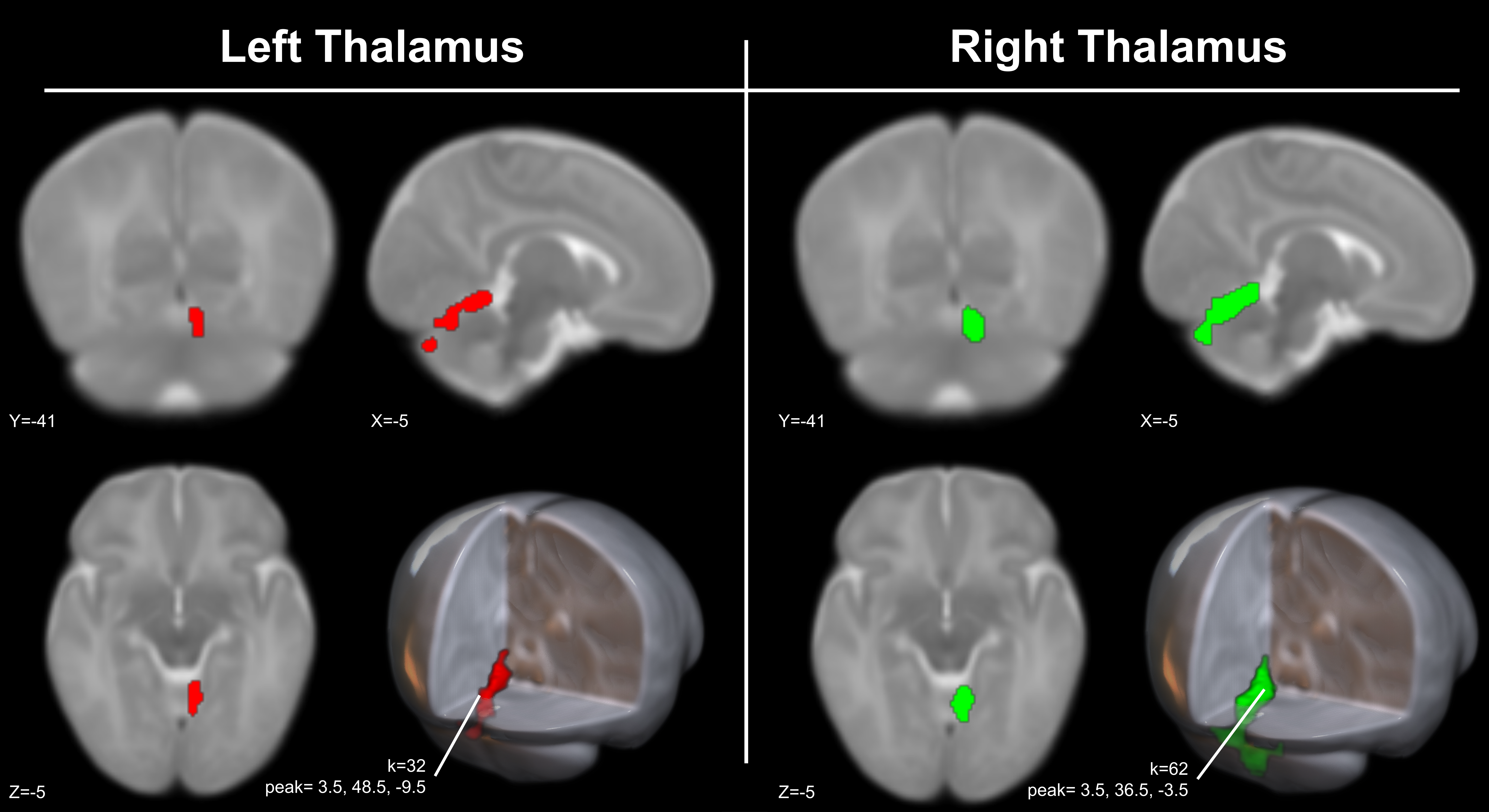
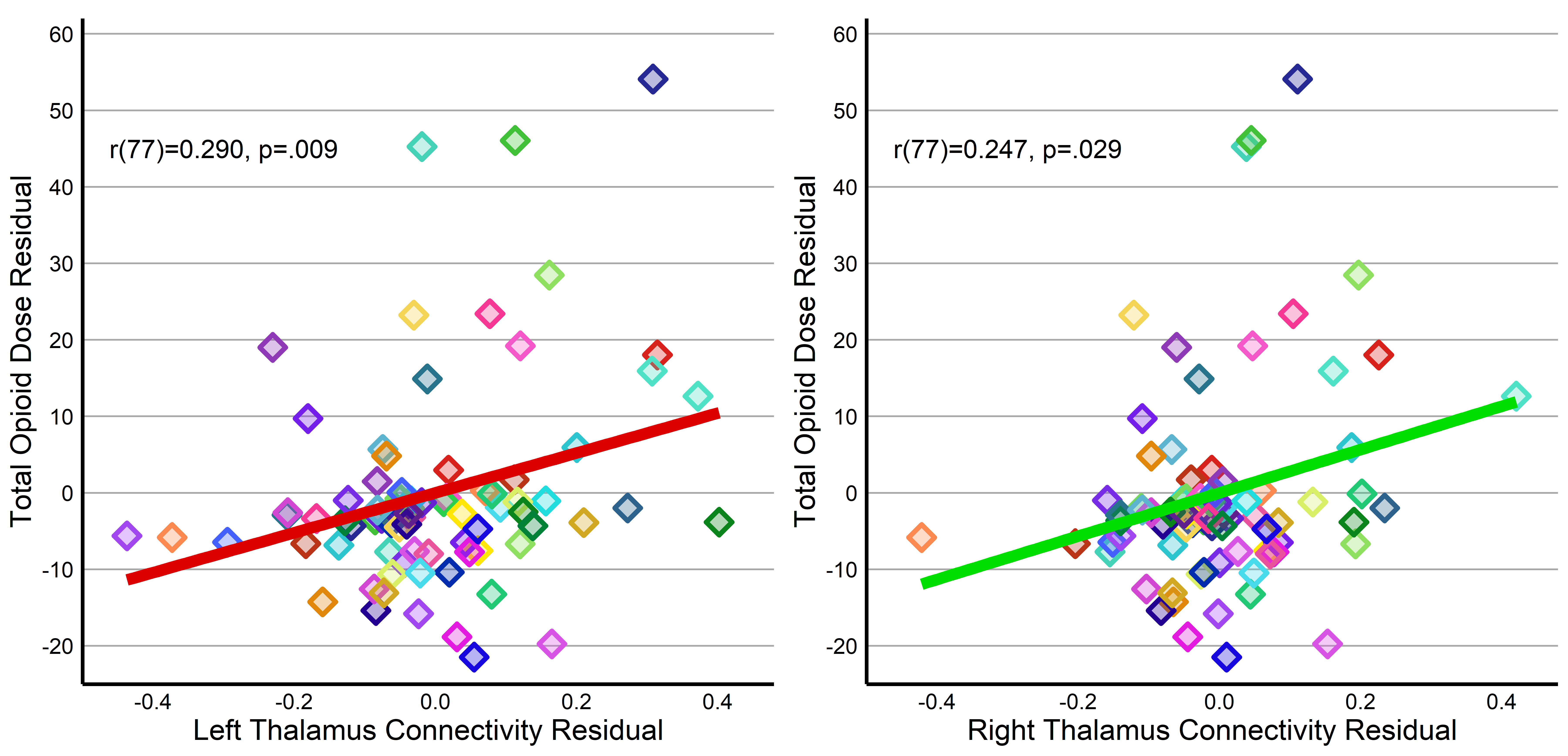
Results: We studied 80 preterm infants who were born at a mean gestational age of 28.1±2.6 weeks. Most infants (n=68) received opioids at least once prior to TEA. Both thalamic seeds yielded significant clusters in the left cerebellum, constituting lobules I-IV, V, and VI (Fig 1). Subsequent partial correlations for thalamic-cluster connectivity with COE, controlling for pain, exhibited positive relationships for the left (r(77)=0.29, p=.009) and right (r(77)=0.25, p=.029) thalamus, suggesting greater COE is associated with stronger connectivity (Fig 2).
Conclusion(s): Our findings suggest that greater COE during NICU admission is associated with an increase in connectivity between the thalamus and cerebellum, after accounting for pain exposures. Importantly, both regions are central to the brain’s pain network and greater connectivity between these two regions has been observed during pain exposures in adults. Our observed hyperconnectivity thus may suggest a possible explanation to later pain sensitivity and motor delays observed in infants exposed to opioids during early life.