Neonatology
Session: Neonatal GI Physiology & NEC 1: Necrotizing Enterocolitis
506 - International Core Outcome Set for Treatment Trials in Necrotizing enterocolitis
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 506
Publication Number: 506.1781
Publication Number: 506.1781

Daphne H. Klerk, Bachelor of Science (she/her/hers)
PhD candidate
University of Groningen, University Medical Center Groningen
Groningen, Groningen, Netherlands
Presenting Author(s)
Background: Many different outcome measures are used in necrotising enterocolitis (NEC) clinical trials, this heterogeneity impairs meta-analyses and the development of evidence-based management guidelines.
Objective: We aimed to develop a Core Outcome Set (COS) for NEC that includes outcome measures most relevant to patients and physicians, from NEC diagnosis to adulthood. This COS is primarily designed for NEC treatment trials, as opposed to diagnostic or prevention studies.
Design/Methods: This study was designed according to COS-STAD recommendations and prospectively registered with the COMET initiative (Study 1920). The protocol has been published (doi: 10.1186/s13063-023-07413-x). We approached >125 clinicians and/or researchers from low-middle and high-income countries based on their scientific contributions to the NEC literature. Patients and parents were approached through local organisations. Participants were separated into two panels, to identify differences in priorities between patients and family representatives and clinicians/researchers involved in and after the neonatal period. All participants were presented with 45 outcomes used in NEC research, identified through a systematic review. To achieve consensus, outcomes were rated on a scale of 1–9 in three online Delphi rounds with feedback from previous rounds and discussed at a final consensus meeting. Consensus was defined as ≥ 70% of participants rating the outcome 7–9.
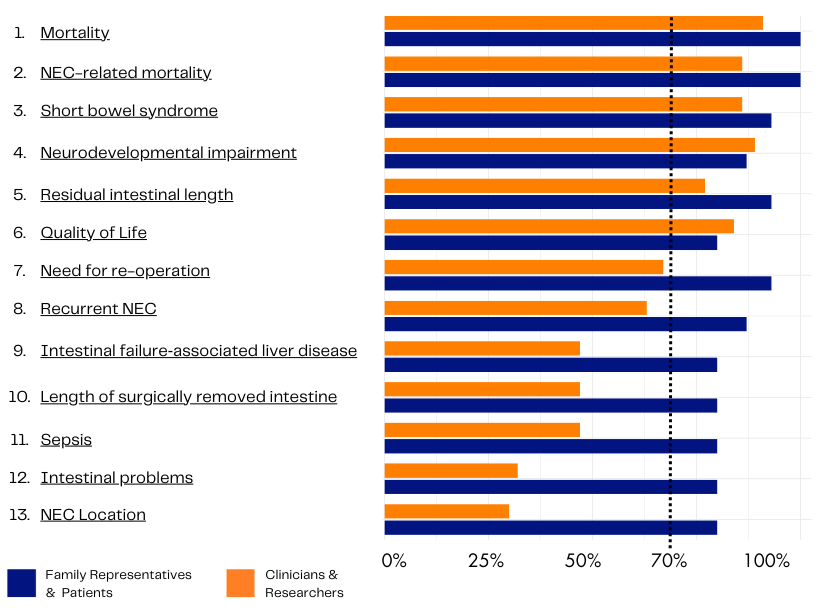
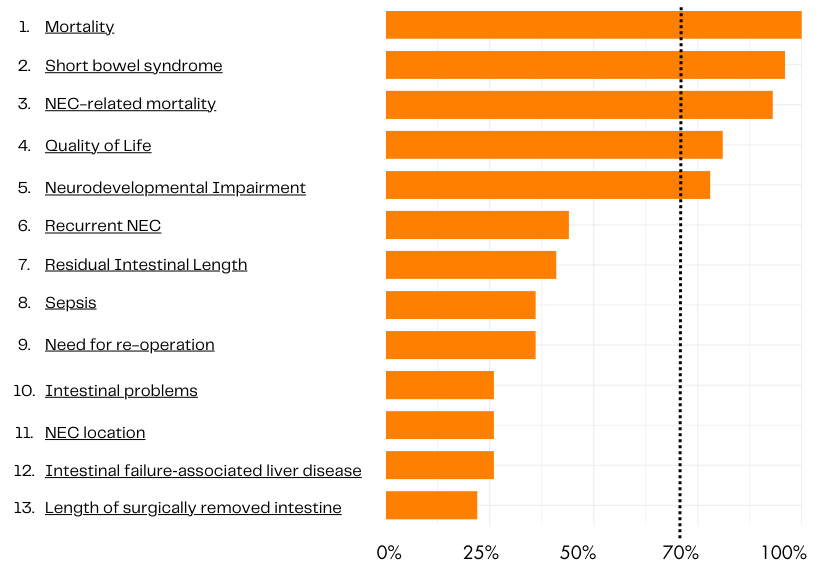
Results: Seventy-one participants from 25 countries, including 15 patients and family representatives, completed all three Delphi rounds. Thirteen outcomes reached consensus in one of the stakeholder groups and were included in the consensus meeting (Figure 1), six outcomes reached consensus in both groups. Twenty-seven participants were present in the online consensus meeting, including family representatives and an individual with personal experience of NEC. In addition, participants from both high- and low-income countries were present. All thirteen outcomes were discussed, but only five outcomes reached consensus after a final vote during the meeting (Figure 2) and were included in the final COS: mortality, NEC-related mortality, short bowel syndrome, quality of life and neurodevelopmental impairment.
Conclusion(s): This core outcome set for NEC trials includes five, predominantly long-term outcomes agreed upon by clinician, patients and family representatives. Use of this international COS will help reduce heterogeneity of outcome reporting in NEC studies, facilitate meta-analyses and ultimately, the care of infants with NEC.