Pediatric Therapeutics and Pharmacology
Session: Pediatric Therapeutics And Pharmacology
516 - Identification of Binding Partners of Inter-alpha Inhibitor Proteins in Human Umbilical Vein Endothelial Cells
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 516
Publication Number: 516.1006
Publication Number: 516.1006

Kazuki Hatayama, MD, PhD (he/him/his)
clinical fellowship
National Center for Child Health and Development, Tokyo, Japan
Tokyo, Tokyo, Japan
Presenting Author(s)
Background: Inter-alpha inhibitory proteins (IAIPs) consisting of heavy chains and a light chain (also termed bikunin) are a family of immunomodulatory inhibitors with anti-inflammatory and neuroprotective effects in neonatal disorders such as hypoxia-ischemia (HI) related brain injury and sepsis. Our previous work revealed a time-dependent entry of exogenous IAIPs but not the bikunin subunit into Human Umbilical Endothelial Cells (HUVECs). Exogenous IAIPs were observed to bind to cell membranes and entered cellular compartments including the cytoplasm and cytosol. IAIPs were also detected in subcellular cytoplasmic structures including endosomes and peroxisomes but not in lysosomes. To determine the molecular partners that facilitate the entry of IAIPs into HUVECs, we investigated interactions and binding of IAIPs with cellular proteins in the cell membranes, cytoplasm, and cytosols.
Objective: To identify the potential molecular binding partners of IAIPs that facilitate entry into HUVECs.
Design/Methods: Membrane, cytoplasmic and cytosolic proteins were isolated and fractionated from HUVECs and enriched on anion exchange chromatographic columns. Fractionated proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes and probed with biotin-conjugated IAIPs along with bikunin or albumin as negative controls. The bound biotinylated proteins were detected by HRP-conjugated streptavidin and visualized by chemiluminescence after several washes. Thereafter, bands identified as positive were cut and sliced from the SDS-PAGE gel after separation and Coomassie brilliant blue staining. The sliced gels were then subjected to LC-MS analysis. Data processing was conducted with MaxQuant. The identified binding proteins were verified with ELISA/solid-phase binding assays.
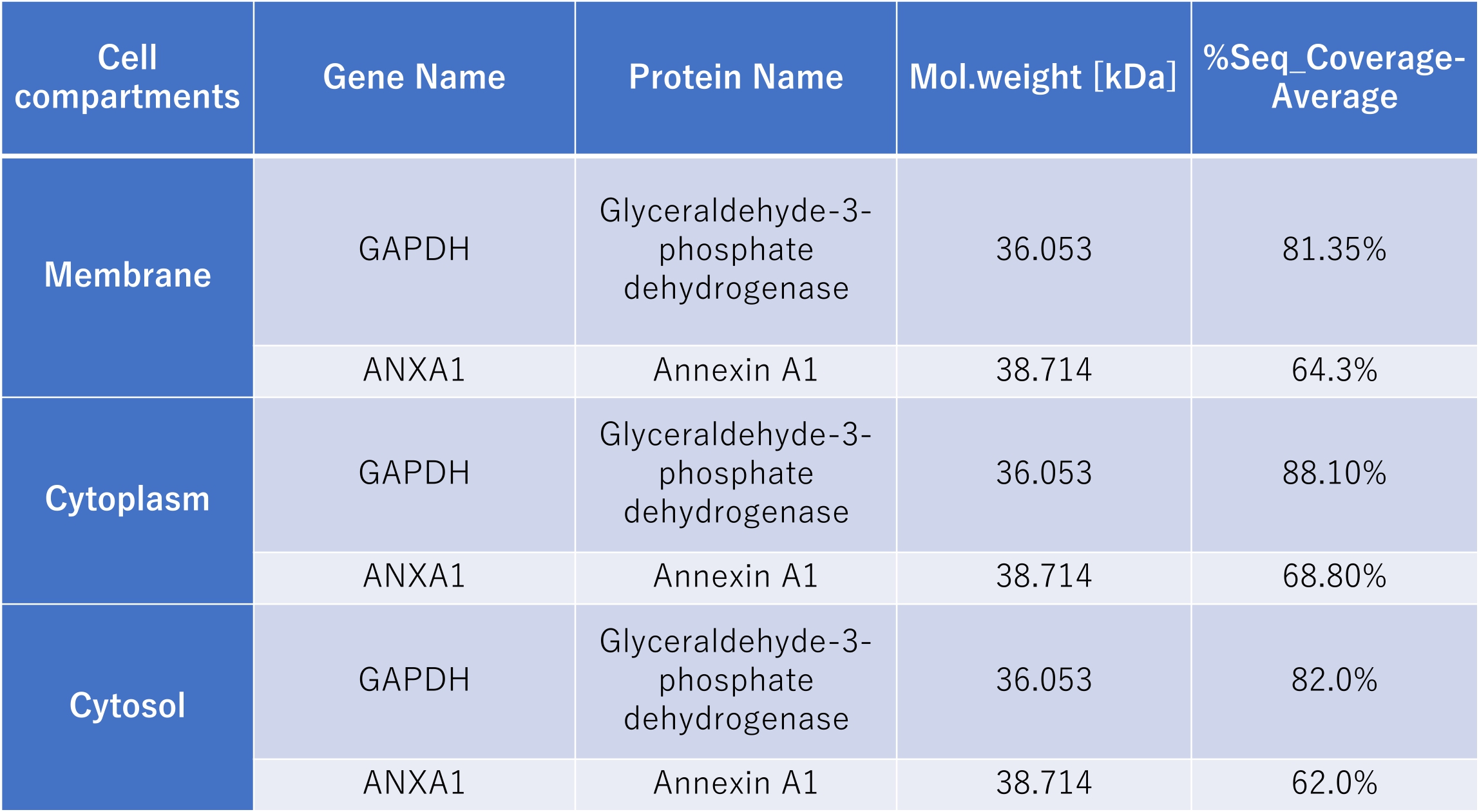
Results: Biotin-conjugated IAIPs were observed to bind strongly to a distinct protein (molecular weight of 37kDa) within the membrane, cytoplasmic, and cytosolic fractions. LC-MS analysis identified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and annexin A1 as the most strongly matched spectral patterns. These proteins were identified in all three fractions (Table 1).
Conclusion(s): GAPDH and annexin A1 have essential functions in many inflammatory disorders such as HI-related brain injury and sepsis. We conclude that the interaction of IAIPs with GAPDH and/or annexin A1 may represent an essential process contributing to the neuroprotective effects of IAIPs in HI-related brain injury and other systemic inflammatory conditions.