Neonatology
Session: Neonatal Pulmonology - Clinical Science 5: New-Old ideas, Stem Cells
574 - Pharmacokinetics and pharmacodynamics after intratracheal all-trans retinoic acid (ATRA) dosing in premature rabbits exposed to hyperoxia
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 574
Publication Number: 574.3160
Publication Number: 574.3160
- GA
Giorgio Aquila, PhD
Senior Scientist
Chiesi Farmaceutici Spa
Parma, Emilia-Romagna, Italy
Presenting Author(s)
Background: Vitamin A (VitA) is an umbrella term for the retinoid family of molecules, which includes retinol, retinoic acid and retinaldehyde. There are two types of retinoid receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs) which, upon binding of retinoid ligands, heterodimerize to control gene expression patterning. Preclinical research showed that RAR/RXR signaling is pivotal in fetal lung development and clinical studies revealed that VitA deficiency in premature infants may increase the risk of bronchopulmonary dysplasia (BPD). Despite clinical evidence showing benefit of VitA supplementation in the prevention of BPD, conflicting data have reduced enthusiasm for VitA supplementation in clinical practice.
Objective: The most biologically active forms of RAR/RXR ligands were explored, focusing on the inhaled approach to maximize lung delivery.
Design/Methods: RAR/RXR selectivity, cell viability and permeability, and lung tissue binding of all-trans retinoic acid (ATRA), AGN195183 (AGN), LGD100268 (LGD) and palovarotene (PALO) were tested in vitro. All of them were investigated for their pharmacokinetics (PK), following intratracheal (IT) dosing in preterm rabbits. ATRA was then tested in premature rabbits exposed to 95% oxygen for 7 days for pulmonary outcomes (lung function and radial alveolar count).
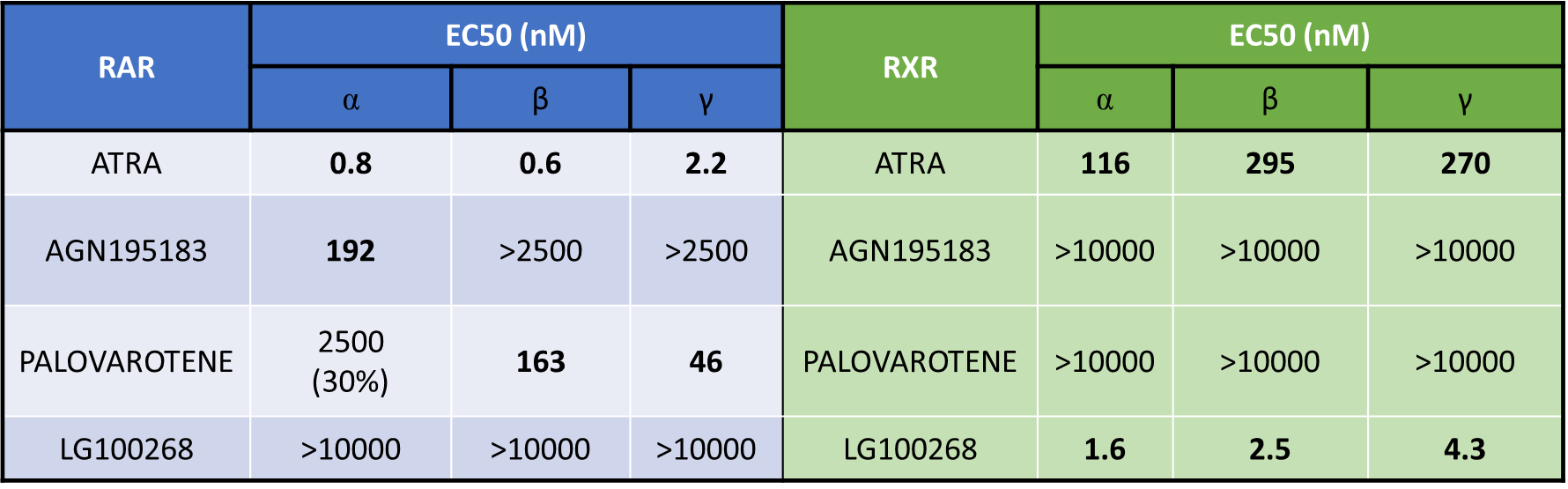
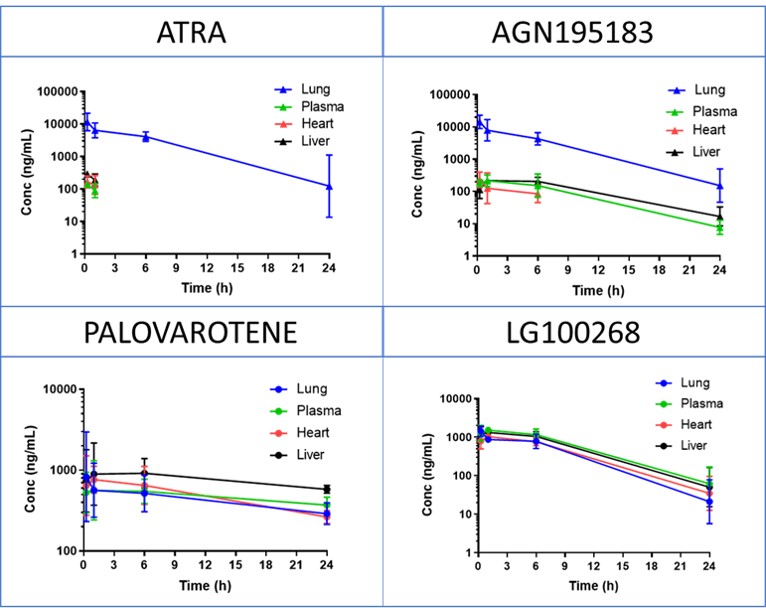
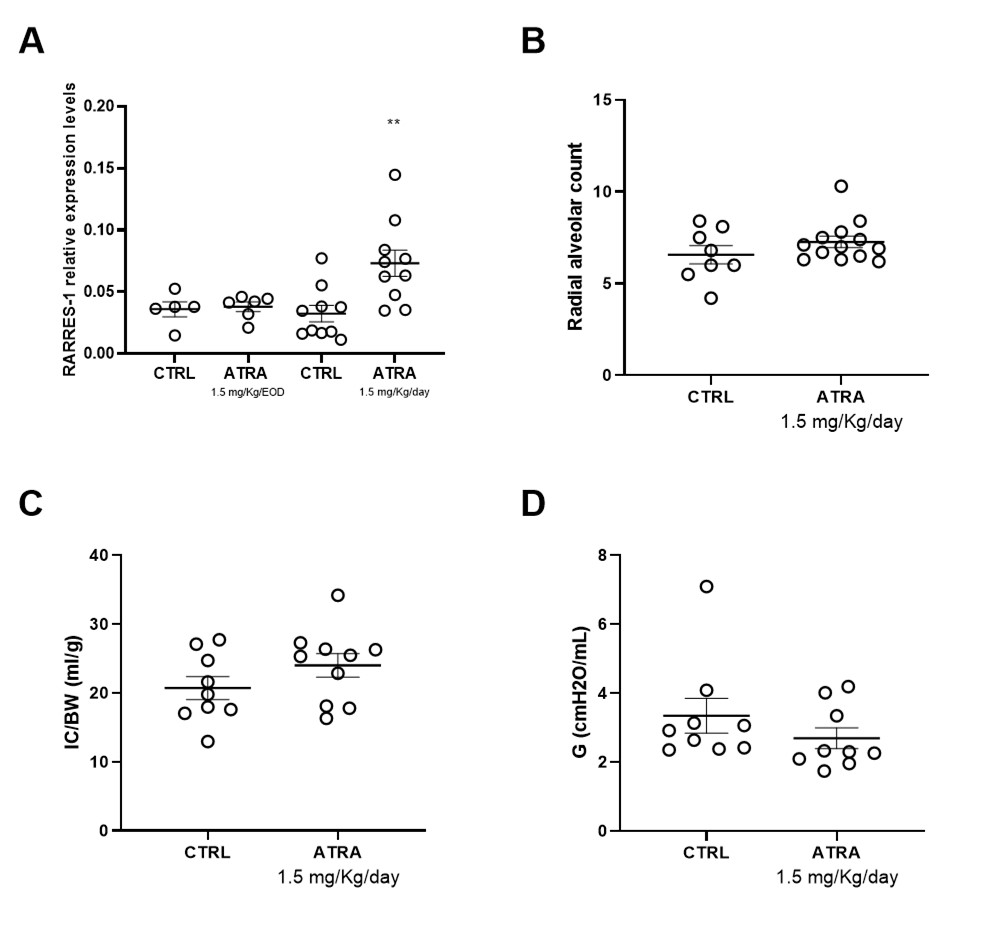
Results: ATRA was identified as pan-RAR/RXR agonist. AGN was classified as RARα agonist, whilst PALO and LGD as RARβ-γ and pan-RXR agonists, respectively (Figure 1). ATRA and AGN showed the lowest epithelial permeability through Caco-2 cells. IT route seemed very promising in enhancing lung exposure of ATRA and AGN only, with the highest lung to plasma partition for ATRA (Figure 2). Only daily IT doses resulted in adequate target engagement (TE), as indicated by increased lung mRNA expression of RARRES-1. Daily ATRA IT administration (1.5 mg/kg) did not significantly improve the pulmonary outcomes in hyperoxia-exposed preterm rabbits (Figure 3).
Conclusion(s): Among several potent retinoids, ATRA was identified as the most promising candidate for intratracheal administration. Further studies are required to find the optimal ATRA posology to maximize RAR/RXR lung targeting and improve lung function.