Neonatology
Session: Neonatal Pulmonology - Clinical Science 6: Respiratory/Neuro Outcomes, Steroids
582 - Salivary Cortisol is Not Associated with Dexamethasone Response in Preterm Infants with Evolving Bronchopulmonary Dysplasia
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 582
Publication Number: 582.3243
Publication Number: 582.3243
- TL
Tamorah Lewis, MD, PhD (she/her/hers)
Associate Professor
University of Toronto Temerty Faculty of Medicine
Toronto, Ontario, Canada
Presenting Author(s)
Background: Preterm infants with evolving BPD receive systemic dexamethasone (DEX), with a goal to reduce pulmonary inflammation, improve lung function, and facilitate weaning from respiratory support. Short-term clinical response to DEX is highly variable and unpredictable. Salivary cortisol may serve as a pharmacodynamic biomarker, helping clinicians identify which infants are most likely to benefit from DEX treatment.
Objective: Define the relationship between baseline and change in salivary cortisol with DEX clinical response.
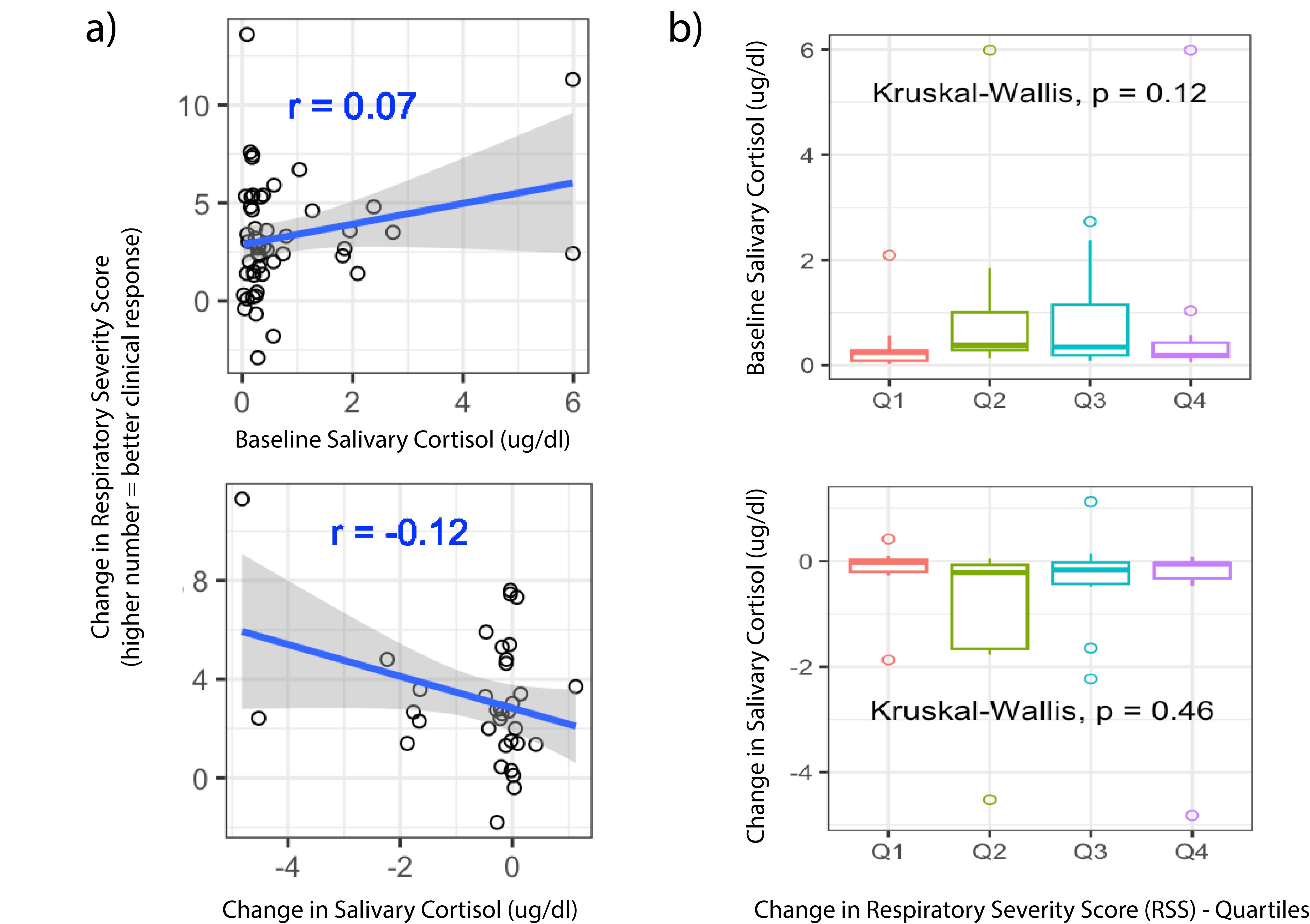
Design/Methods: In a multi-site prospective cohort study, preterm infants who were receiving DEX for 7-10 days for BPD were enrolled for sample and data collection. Salivary cortisol was measured prior to the first dose of DEX and then a second time, on days 5-8 of treatment. When available, multiple samples in 24 hrs were averaged for the “pre” and “post” cortisol for each infant. DEX clinical response was quantified by change in Respiratory Severity Score (RSS=Fi02xMAP). Cortisol was quantified by Salimetrics per high sensitivity enzyme immunoassay (μg/dL). Statistical analysis (Spearman correlations, Kruskall- Wallis testing and linear regression) was performed in R.
Results: A total of 54 infants who underwent their first dexamethasone exposure were included in this analysis. All 54 had baseline cortisol, and 38 had “post” samples for analysis. Median (IQR) gestational age 25.1 (24.1,26.5) weeks, birthweight 742 (591,890) grams, day of life DEX started 30 (23,48) days. The median change in RSS at day 7 was 2.7 (1.4,4.8) with higher numbers indicating better response. The “post” cortisol sample was collected at 6.8 (5.9,7.6) days of treatment. Baseline cortisol was 0.3 (0.2,0.6) ug/dl or 8.3 (5.5,16.5) nmol/L, “post” cortisol was 0.2 (0.1,0.3) ug/dl or 5.5 (2.8,8.3) nmol/L and change in cortisol was -2.8 (-11.0,0) nmol/L. There was no statistically significant correlation between change in RSS and (1) baseline cortisol, (2) change in cortisol level (Figure 1). When comparing lowest quartile and highest quartile of DEX response, there was no significant difference in baseline cortisol (p=0.8) or change in cortisol (p=0.78).
Conclusion(s): To our knowledge, this is the first study to assess salivary cortisol as a predictive or response biomarker for DEX in BPD. Based on the results of this small cohort of DEX treated infants, baseline salivary cortisol does not predict dexamethasone response. Decrease in salivary cortisol is not statistically associated with degree of DEX response, but this finding could be spurious due to limited sample size.