Immunizations/Delivery
Session: Immunizations/Delivery 1
35 - Six-Month Safety and Immunogenicity of mRNA-1273.214 Booster Vaccination in Individuals Aged 6 Months to <6 Years: Interim Results From a Phase 3, Open-Label Trial
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 35
Publication Number: 35.1497
Publication Number: 35.1497
- AD
Avika Dixit, MBBS MPH MBI (she/her/hers)
Director, Clinical Development
Moderna, Inc.
Cambridge, Massachusetts, United States
Presenting Author(s)
Background: A 2-dose primary series of the original mRNA-1273 vaccine induced durable neutralizing antibody (nAb) responses against ancestral SARS-CoV-2 in children 6 months to 11 years of age.
Objective: To present interim results from a phase 3 study evaluating 6-month safety and durability of immune responses after an omicron-BA.1 variant-containing (mRNA-1273.214) booster dose among children aged 6 months to 5 years.
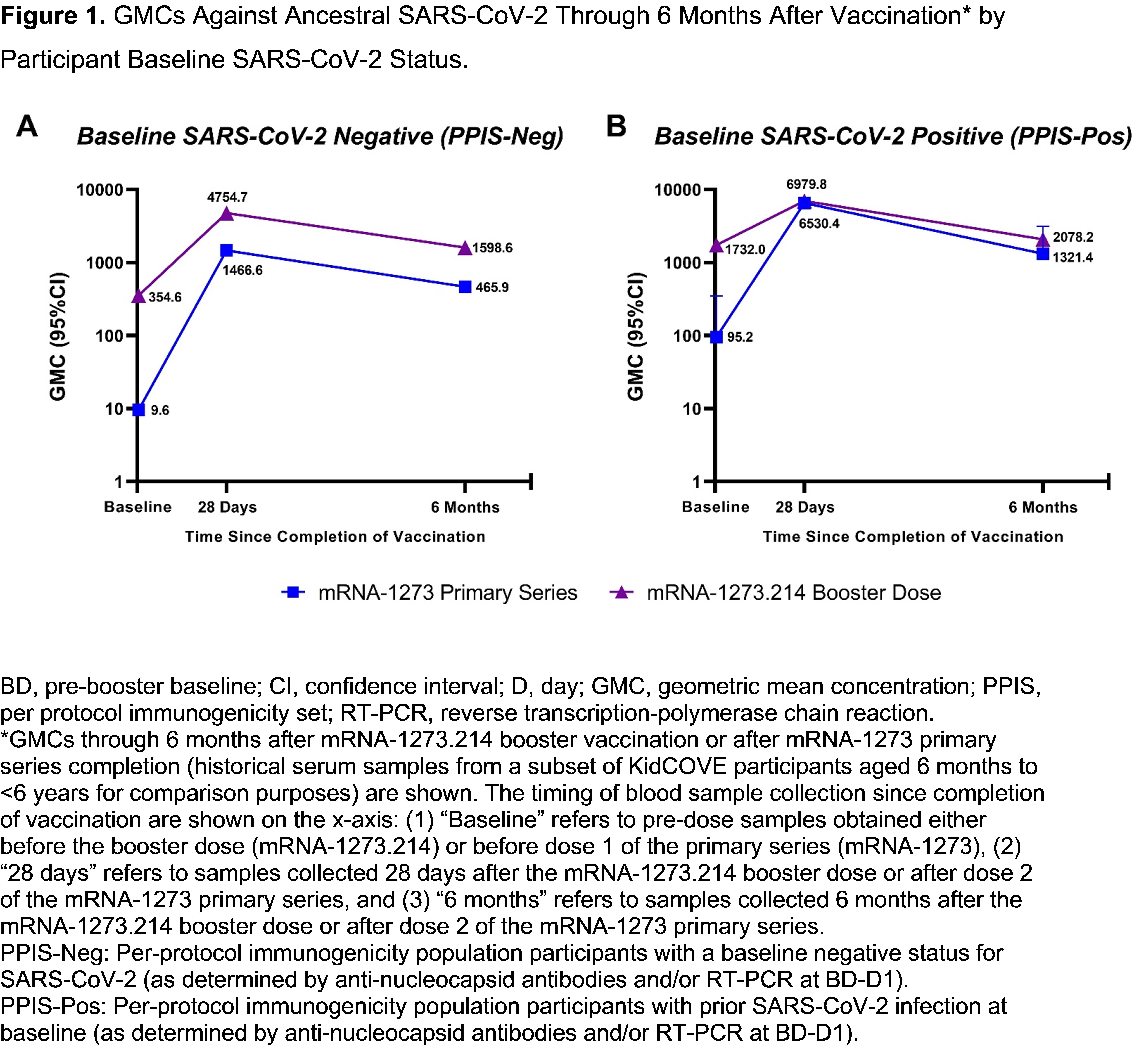
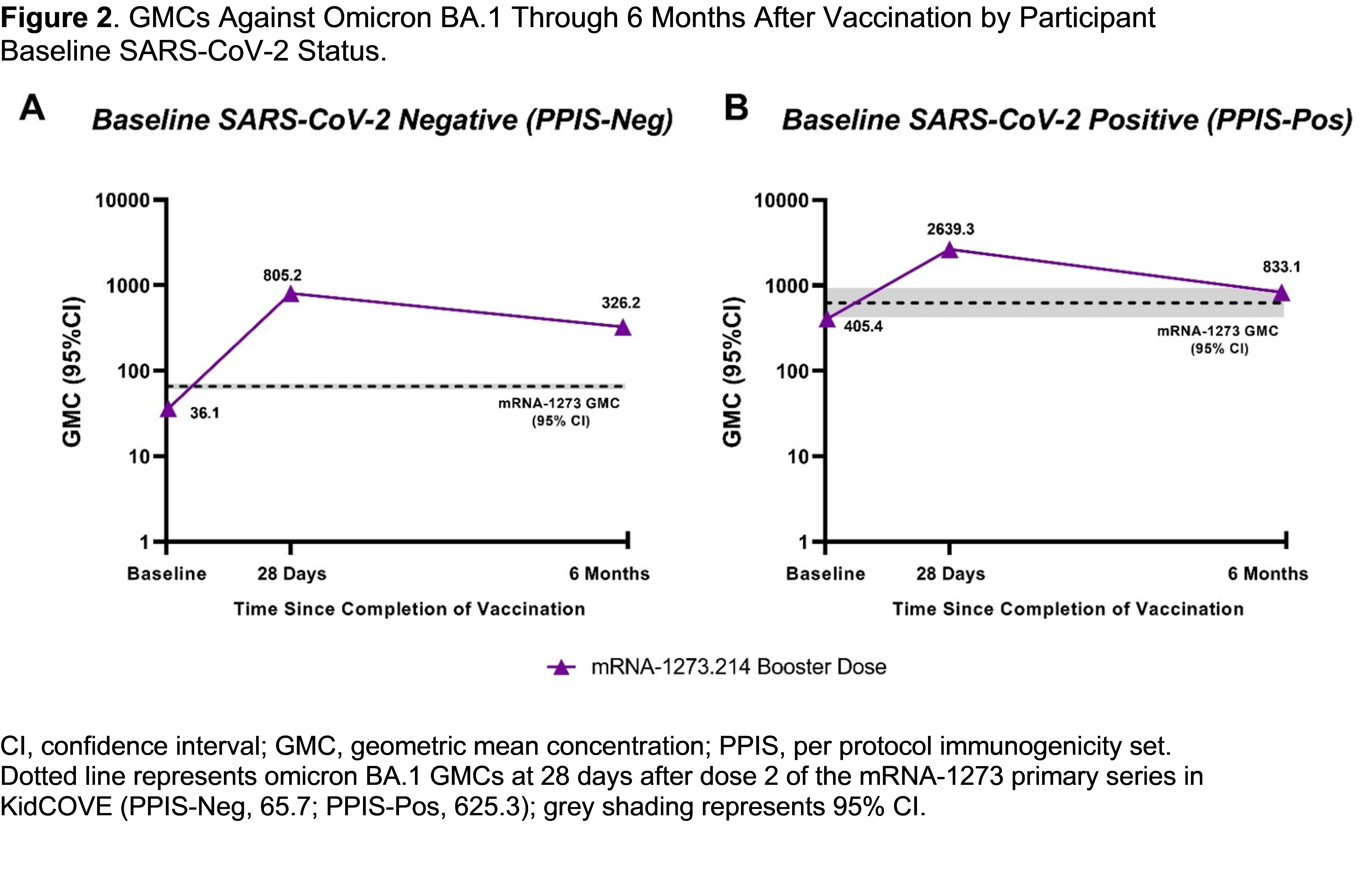
Design/Methods: This open-label trial evaluated an mRNA-1273.214 booster dose (10 µg) administered to participants aged 6 months to 5 years who received the 2-dose mRNA-1273 (25 µg) primary series in the KidCOVE trial ≥4 months prior (NCT05436834). Safety was assessed through 6 months after vaccination. Immunogenicity was assessed as geometric mean concentrations (GMCs; with 95% confidence intervals [CIs]) of nAbs against ancestral SARS-CoV-2 and omicron (BA.1) measured at pre-booster baseline (BD-D1) and post-booster at 28 days (BD-D29) and 180 days (BD-D181; Month 6).
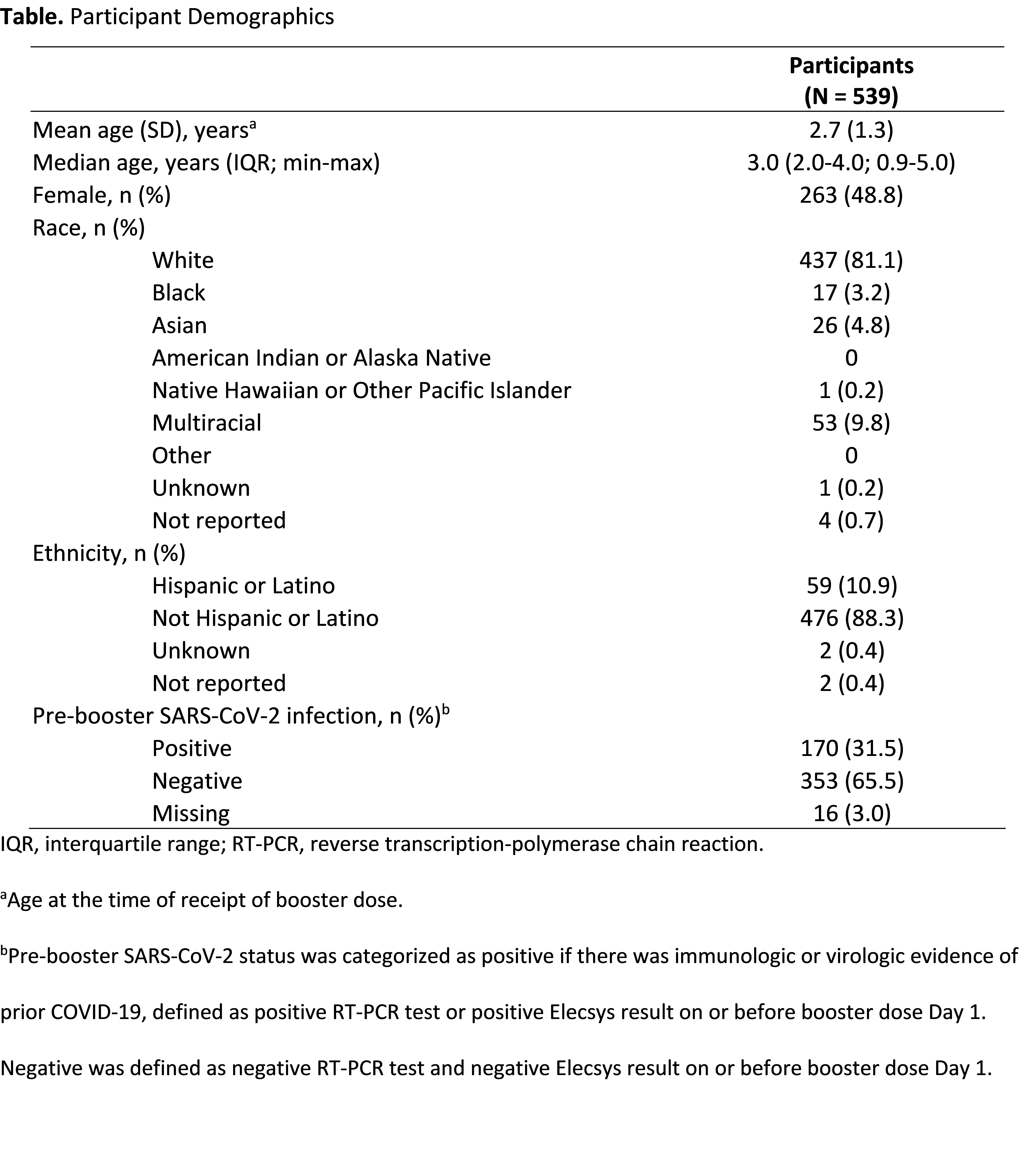
Results: There were 539 mRNA-1273.214 booster recipients included in this interim analysis; median age was 3 years, and most participants were male (Table). At 6 months post-booster, mRNA-1273.214 had a similar safety profile as the mRNA-1273 primary series and was generally well-tolerated. Incidence of serious adverse events (SAEs) was low (1.7%) and none were vaccine-related; 4 adverse events of special interest were reported, of which 1 (erythema multiforme on BD-D2) was considered related. Among participants who were negative for SARS-CoV-2 at baseline, an mRNA-1273.214 booster induced high nAb responses against ancestral SARS-CoV-2 (Figure 1) and BA.1 (Figure 2) at BD-D29 that remained elevated through 6 months post-booster (GMCs [95% CI], ancestral: pre-booster, 354.6 [328.4-382.8]; BD-D29, 4754.7 [4327.3-5224.3]; BD-D181, 1598.6 [1420.7-1798.9]; BA.1: pre-booster, 36.1 [33.0-39.5]; BD-D29, 805.2 [718.2-902.8]; BD-D181, 326.2 [283.0-376.1]). Similar trends were observed in participants who were positive for SARS-CoV-2 at baseline.
Conclusion(s): Long-term safety data of a BA.1 variant-containing booster (mRNA-1273.214) in children aged 6 months to 5 years reflected events typical for this age group, with no vaccine-related SAEs. The booster induced durable immune responses above pre-booster baseline against both ancestral and BA.1 strains. This study was funded by Moderna, Inc.