Neonatology
Session: Neonatal Clinical Trials 1
467 - Population Pharmacokinetics of Caffeine in Neonates with Hypoxic-Ischemic Encephalopathy
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 467
Publication Number: 467.1680
Publication Number: 467.1680
.jpg)
Elizabeth J. Thompson, MD (she/her/hers)
Fellow
Duke
Durham, North Carolina, United States
Presenting Author(s)
Background: Hypoxic-ischemic encephalopathy (HIE) due to perinatal asphyxia is common and often fatal. Therapeutic hypothermia improves outcomes yet ~30% of neonates with HIE die or have neurodevelopmental impairment. Caffeine as an adjuvant therapy may offer neuroprotection to neonates with HIE by blocking adenosine receptors in the brain and reducing neuronal cell death. The effects of hypothermia on caffeine pharmacokinetics (PK) are unknown.
Objective: We aimed to characterize caffeine PK in neonates with HIE receiving therapeutic hypothermia.
Design/Methods: We performed an open-label, non-randomized, dose escalating PK and safety trial of caffeine in neonates with HIE. We included neonates born ≥36 weeks gestational age and receiving therapeutic hypothermia. All neonates received a 20 mg/kg loading dose of caffeine in the first 24 hours of life followed by up to 2 daily doses of 5 mg/kg (n=9) or 10 mg/kg (n=8). We performed a population PK analysis using NONMEM. The effects of clinical covariates, including cooling, on PK parameters were evaluated. Dosing simulations were conducted to target concentrations of 15-25 mg/L for apnea of prematurity efficacy and < 46 mg/L for safety.
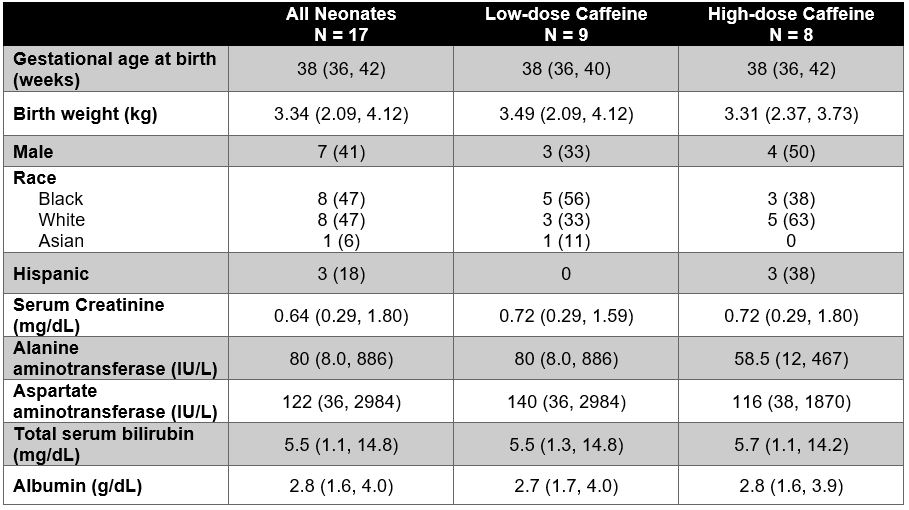
Results: A total of 17 neonates were enrolled and 112 PK samples were collected. Cohort characteristics are shown in Table 1. Two neonates withdrew from the study early: one for withdrawal of care and one for new seizure activity. A 1-compartment model best fit the data and is described by the following equations:
CLi (L/h/70kg)=0.445 * (WTi/70)^0.75
Vi (L/70kg)=87.1 * (WTi/70)
Population parameters are shown in Table 2. Cooling was not a significant covariate in our small cohort of neonates. In a virtual population of 1000 neonates, average steady state concentrations were simulated (Figure 1). In the low dose group, 53% of simulated observations were below the therapeutic level of 15 mg/L and none were above the safety threshold of 46 mg/L. In the high dose group, 5.3% of simulated observations were below the therapeutic level and only 0.05% of concentrations were above the safety threshold.
Conclusion(s): A one compartment model best described caffeine PK in term neonates undergoing cooling for HIE. Weight-normalized CL was similar to previously reported values in preterm neonates, but weight-normalized V was ~25% larger. High dose caffeine resulted in more therapeutic exposures without significant safety concerns, although exposure targets are not yet defined for neuroprotection in HIE. Further studies should explore if higher caffeine exposures improve outcomes without increased adverse events.
.jpg)
.jpg)
