Neonatology
Session: Neonatal Clinical Trials 1
468 - A Randomized Trial Utilizing Urine Sodium Concentrations to Guide Sodium Supplementation in Preterm Neonates
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 468
Publication Number: 468.1304
Publication Number: 468.1304

Brianna Liberio, MD (she/her/hers)
Assistant Professor of Clinical Pediatrics
Indiana University School of Medicine
Indianapolis, Indiana, United States
Presenting Author(s)
Background: Sodium (Na) homeostasis is vital to optimal growth. Many preterm infants may experience Na depletion as providers fail to account for the extent of urine Na losses. Resultant suboptimal postnatal growth may be associated with adverse neurodevelopment. In a cohort study, use of a physiologic-based algorithm to identify Na-deficient preterm infants using urine Na concentrations (UNa) to guide Na supplementation resulted in significantly greater postnatal weight gain. A randomized controlled trial is needed.
Objective: Determine the effects on somatic growth parameters of adherence to a clinical practice algorithm utilizing UNa to guide Na supplementation as compared to current practices in a randomized, controlled trial.
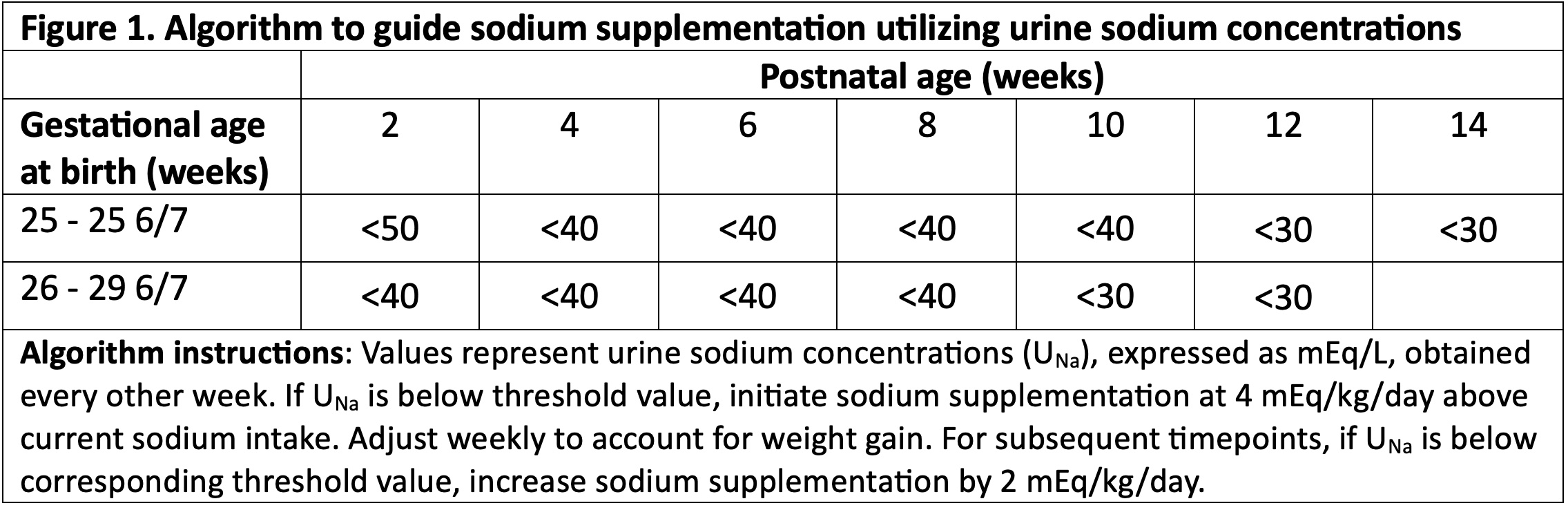
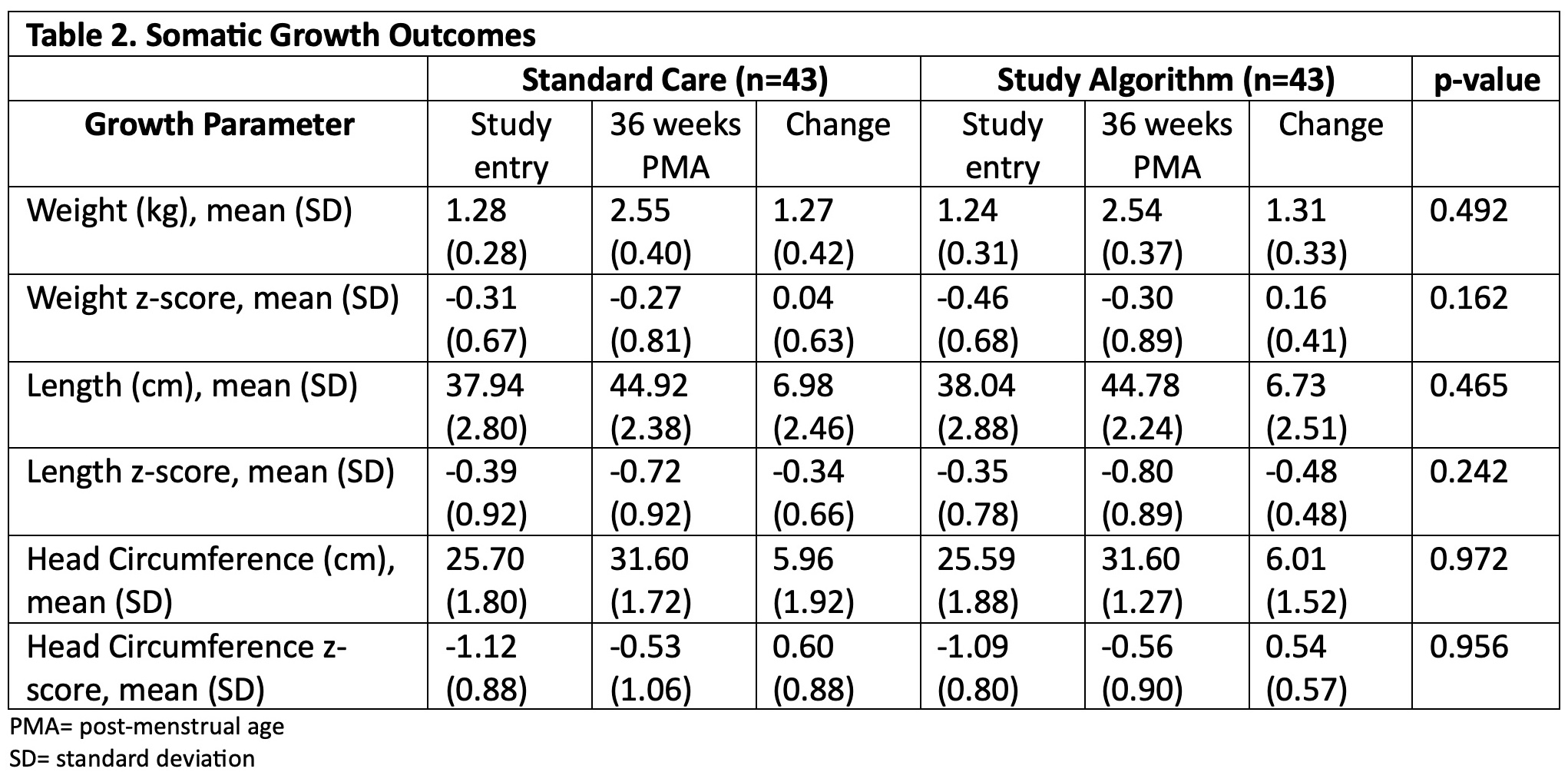
Design/Methods: Prospective trial randomizing infants to an algorithm using UNa every two weeks to guide Na supplementation (NaSupp) or standard care (SC) (Figure 1). Infants with birth gestational age (GA) 25 0/7 – 29 6/7 weeks, birth weight >/=500 grams, admitted in the first week of life, and < 17d of age were eligible. Those with major congenital anomalies, kidney dysfunction, diuretic use, or ostomies were excluded. Primary outcome was somatic growth (weight, length, head circumference) evaluated by the change in Z-score between 2 weeks of age and 36 weeks post-menstrual age (PMA) or transfer from NICU.
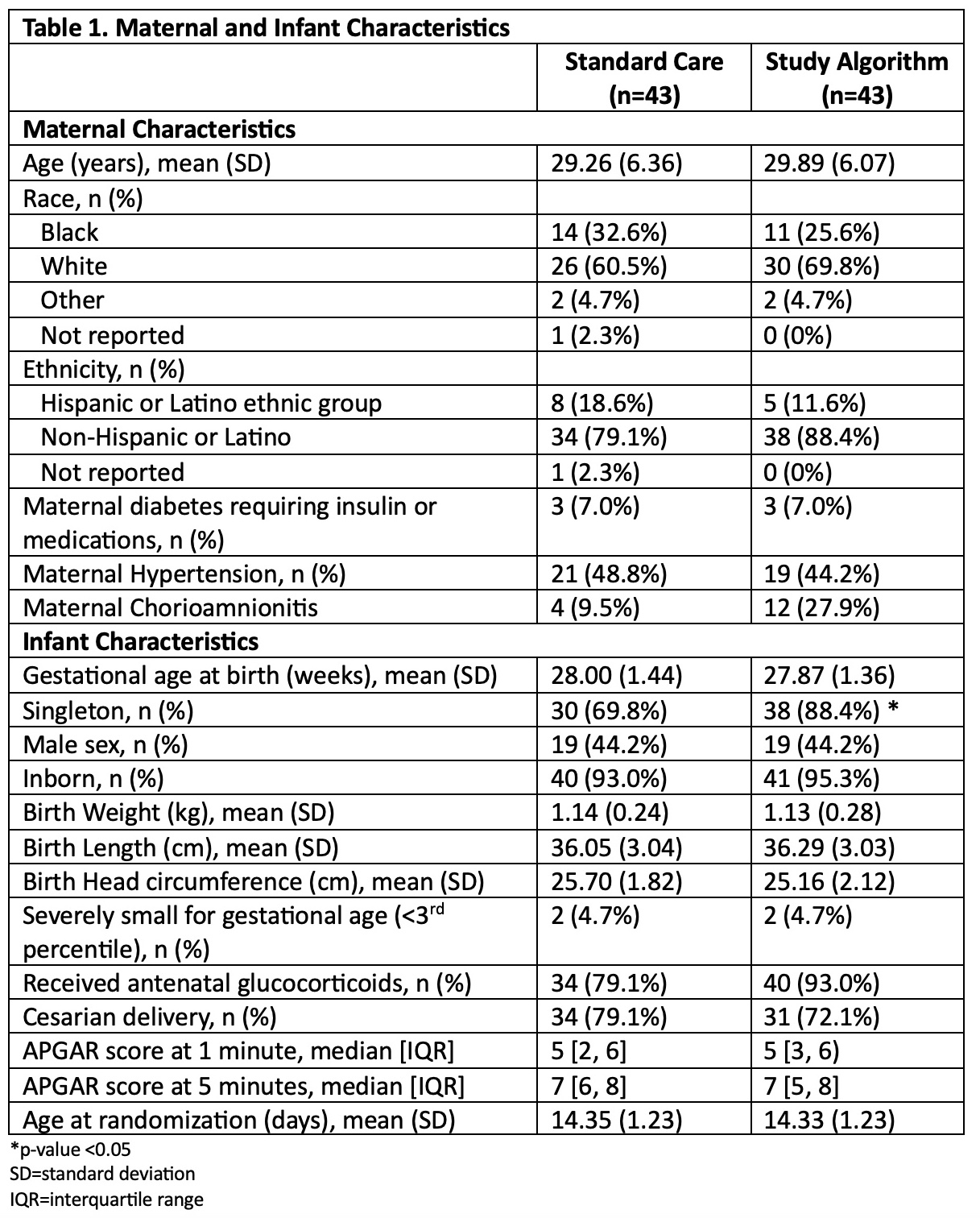
Results: 90 infants were randomized, 86 infants (43 each arm) were included in the analysis. Maternal and infant characteristics were similar between arms. Average GA at birth, birthweight, and daily calorie receipt were similar (Table 1). NaSupp infants received more Na than SC (5.75 (SD 0.96) vs 3.62 (SD 1.25) mEq/kg/d, p< 0.001). There were no differences in receipt of human milk vs formula. 25 (58.1%) NaSupp infants had an initial UNa < 40 mEq/L, and 38 (88.4%) infants had a low UNa at some timepoint. There were no significant differences between groups in change in Z-score for weight, length, or head circumference between study entry and 36 weeks PMA (Table 2). However, NaSupp infants >28 weeks GA demonstrated an increase in absolute weight gain compared to SC. There were no differences in measured morbidities. Total body water and energy expenditure measurements are ongoing.
Conclusion(s): Despite successfully implementing a clinical practice algorithm utilizing UNa to guide Na supplementation in preterm infants, there were no significant differences in somatic growth outcomes at 36 weeks PMA in NaSupp infants compared to SC. Further refinement of ways to identify preterm infants at risk for growth failure associated with Na depletion are needed.