Neonatology
Session: Neonatal Fetal Nutrition & Metabolism 2: Fetal/Neonatal Metabolic Dyscrasias
128 - The Impact of Maternal Diabetes in Pregnancy and Preterm Birth on Energy Metabolism Hormone Concentrations in Infants
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 128
Publication Number: 128.323
Publication Number: 128.323
.jpg)
Catherine O. Buck, MD (she/her/hers)
Assistant Professor of Pediatrics
Yale School of Medicine
Guilford, Connecticut, United States
Presenting Author(s)
Background: Preterm birth and exposure to diabetes (DM) in pregnancy are independent risk factors for the development of metabolic disease. Fetal reprogramming is a potential driver of this association, and energy metabolism hormones, or adipokines, may be markers of adiposity development. However, changes in adipokine concentrations during early infancy are poorly characterized, especially among preterm infants.
Objective: To characterize changes in adipokine concentrations in first postnatal week, including the influence of preterm birth and exposure to DM on these concentrations.
Design/Methods: Term and preterm (30 0/7 - 36 6/7) infants born at a tertiary care urban academic hospital from 2020 to 2022 were enrolled after birth. Concentrations of leptin, adiponectin, insulin, and resistin were measured in cord and infant serum samples collected from birth through postnatal day 7. Linear mixed effects models were used to assess the impact of postnatal day (using polynomial effects to test for change in slope over time), preterm birth, maternal DM and their interaction on the natural log of the adipokine concentrations.
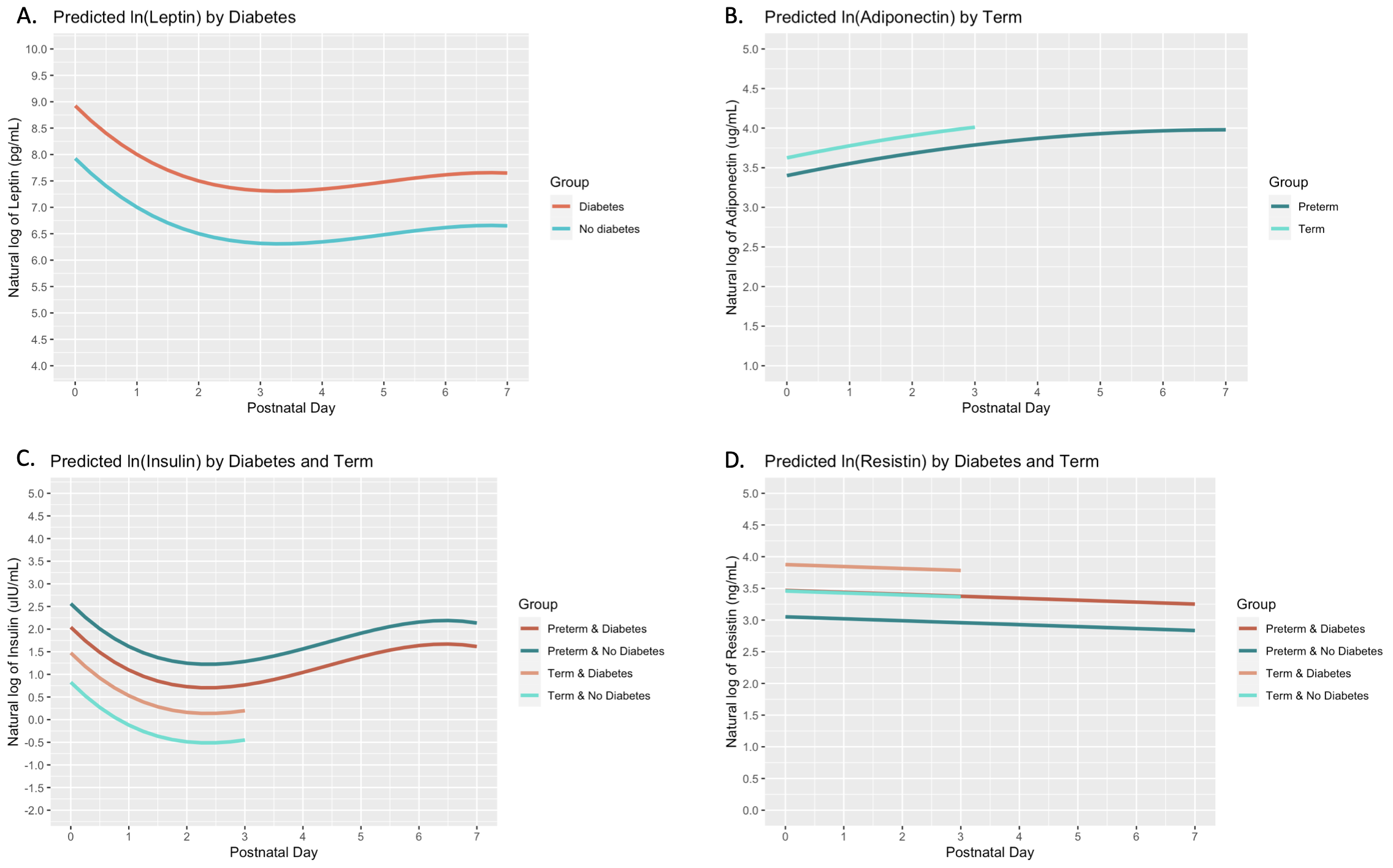
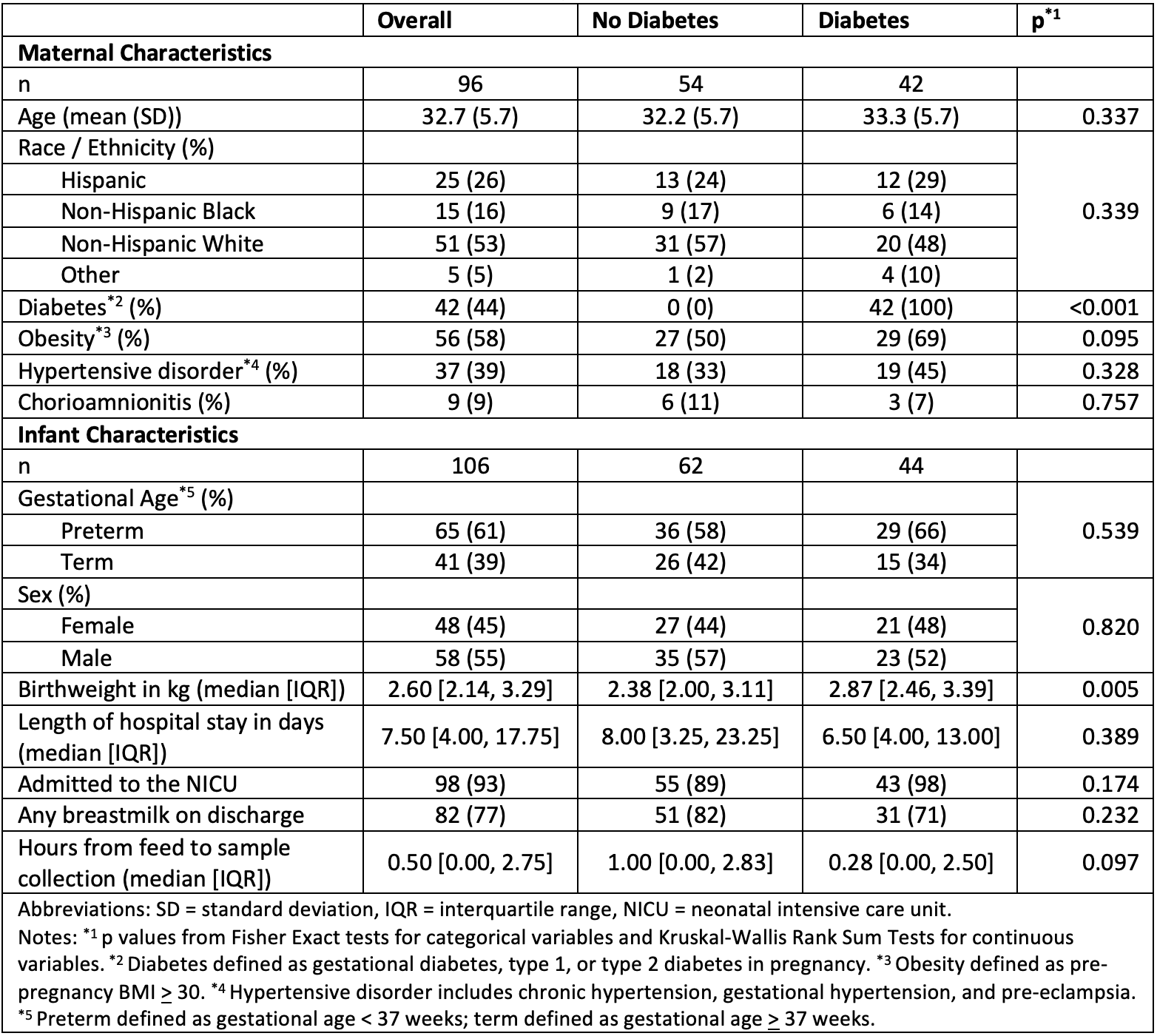
Results: Among 106 infants included in the study, 65 (61%) were born preterm and 62 (58%) were exposed to DM. Infants in the DM group had a higher birthweight (median 2.87 vs 2.38 kg, p=0.005) compared with infants in the non-DM group (Table 1). Leptin concentrations dipped from birth to day 3, followed by a modest rebound through day 7, and were on average higher among infants in the DM group (Table 2, Figure 1). Adiponectin concentrations increased with an attenuated slope from birth through day 7, and were higher among term infants. Insulin concentrations showed an initial dip and a comparable rebound by day 7, and were related to DM exposure, preterm birth, and their interaction, with the highest concentrations in the non-DM preterm group. Resistin concentrations decreased linearly with time and were higher in the DM and term groups.
Conclusion(s): In this cohort of term and preterm infants, adipokine concentrations showed dynamic changes over the first postnatal week. These suggest unique signatures in infant leptin and resistin related to DM exposure, in adiponectin and resistin related to prematurity, and a combination of both DM exposure and prematurity on insulin. The influence of maternal metabolic health in pregnancy on adipokine trajectories may reflect infant metabolism development. Future studies to evaluate associations of adipokine trajectories with newborn adiposity measures will inform the use of these hormones as biomarkers of later cardiometabolic health in preterm infants.
.png)