Health Services Research
Session: Health Services Research 2: Novel Methods and Disparities
301 - FDA Approval of Therapeutic Medical Devices for Use in Pediatric Patients, 2008 - 2020
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 301
Publication Number: 301.1529
Publication Number: 301.1529

Ryan Brewster, MD (he/him/his)
Resident Physician
Boston Children's Hospital
Boston, Massachusetts, United States
Presenting Author(s)
Background: Unique and multifactorial barriers have limited the advancement of medical devices compared to adults, leading to off-label use of devices without adequate safety and efficacy data. Enacted in 2007, the Pediatric Medical Device Safety and Improvement Act (PMDSIA) was the first legislative effort to support pediatric device development. Among its provisions, the law allocated targeted funding and stipulated improved monitoring of regulatory activities pertaining to pediatric device labeling.
Objective: To examine characteristics of and trends in pediatric therapeutic medical device approvals since passage of PMDSIA.
Design/Methods: We conducted a cross-sectional analysis of novel therapeutic pediatric medical devices from 2008-2020 using annual reports submitted to Congress by the FDA to describe pediatric device approval activities. Detailed in the reports are products that the FDA has deemed pertinent to, but not approved for, a pediatric subpopulation, allowing for an estimation of the innovation gap. Diagnostic medical devices were excluded. Yearly proportions of devices approved for pediatric use were calculated relative to the number of devices determined by the FDA to be relevant to children. Temporal trends were assessed with univariate logistic regression.
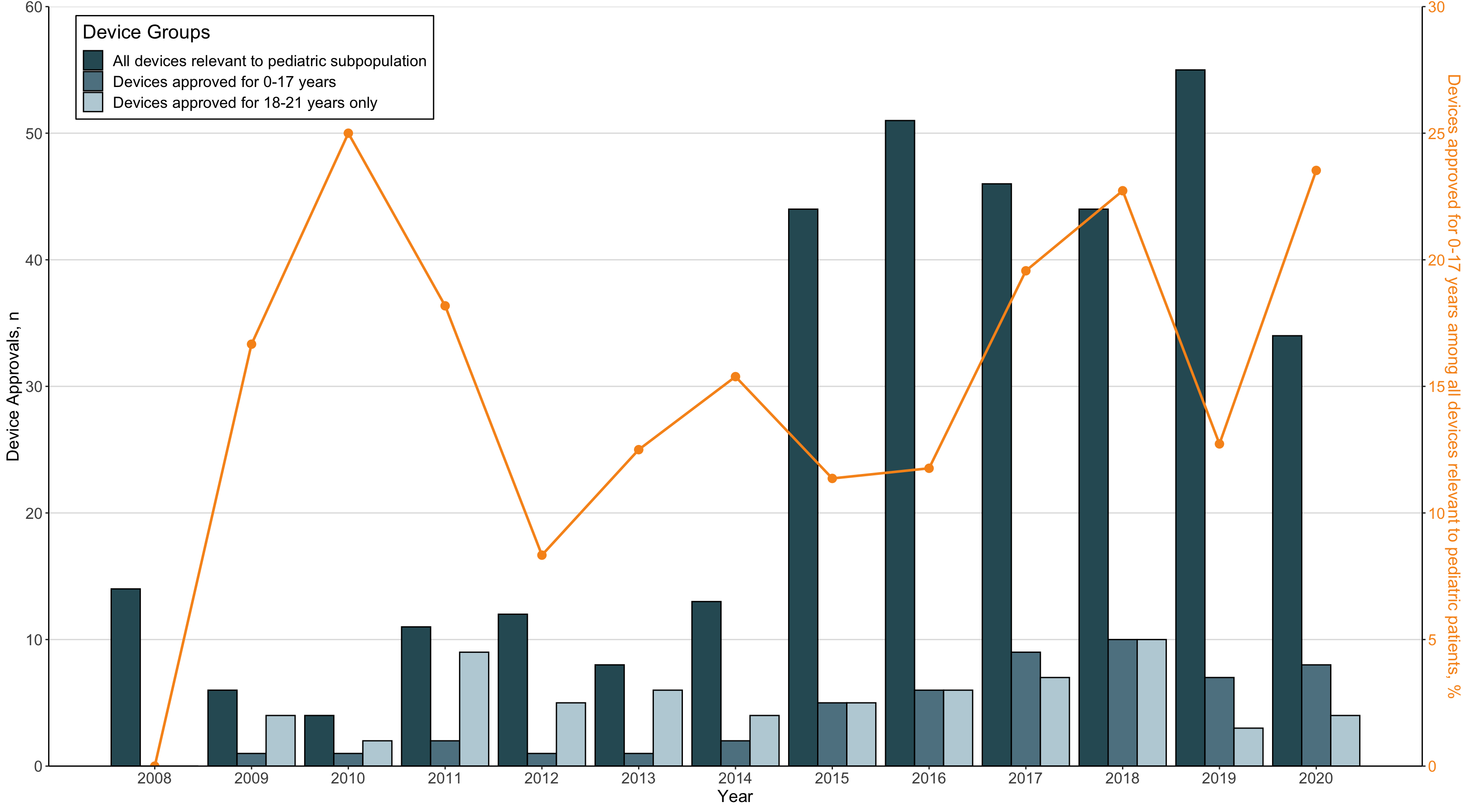
Results: There were 473 therapeutic medical devices approved from 2008-2020, of which 342 (72.3%) were deemed relevant to a pediatric subpopulation. A total of 118 therapeutic devices were approved with a pediatric indication, corresponding to 34.5% of relevant devices (Table 1). Among these pediatric devices, 54 (45.7%) were approved for use in children 0-17 years and 64 (54.2%) for individuals 18-21 years only (Figure 1). Overall, 15.8% (54/342) of relevant devices were approved in children 0-17 years. Most pediatric devices were approved via the PMA pathway (90.7%) and target cardiovascular (32.2%) and endocrine (22%) conditions. The annual proportion of devices approved for children 0-17 years among devices relevant to pediatric subpopulations remained unchanged (OR: 1.09, 95% CI: 0.99-1.22)
Conclusion(s): Despite promising federal mandates, low rates of therapeutic medical device approvals for children have persisted since the PMDSIA with limited improvement over time. Only 15.8% of products were labeled for patients 0-17 years, the majority of which focused on just two therapeutic areas, suggesting even greater disparities than previously estimated. Our results highlight that to align with the original legislative intent, the FDA should provide pediatric age categories that conform to conventional clinical definitions.
.png)