Neonatology
Session: Neonatal Neurology 6: Clinical
556 - Gabapentin Use in 412 Neonatal Intensive Care Units between 2005 and 2020
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 556
Publication Number: 556.1615
Publication Number: 556.1615
.jpg)
Rachel G. Greenberg, MD, MB, MHS (she/her/hers)
Associate Professor
Duke Clinical Research Institute
Durham, North Carolina, United States
Presenting Author(s)
Background: Gabapentin is a gamma-aminobutyric acid analog with antinociceptive, antiepileptic and anxiolytic properties targeting pathways involving neuropathic pain and inflammation. It is currently FDA approved for postherpetic neuralgia, partial seizures, and restless leg syndrome in adults and partial epilepsy in children 3 years and older. There are no FDA-approved indications for use in infants and research surrounding infant use is currently limited. Despite this, studies have shown a rise in off-label gabapentin use in Neonatal Intensive Care Units (NICUs) across the US.
Objective: To analyze the trends of gabapentin use in US NICUs and examine the associations of use with demographic characteristics, other diagnoses, and concomitant medications.
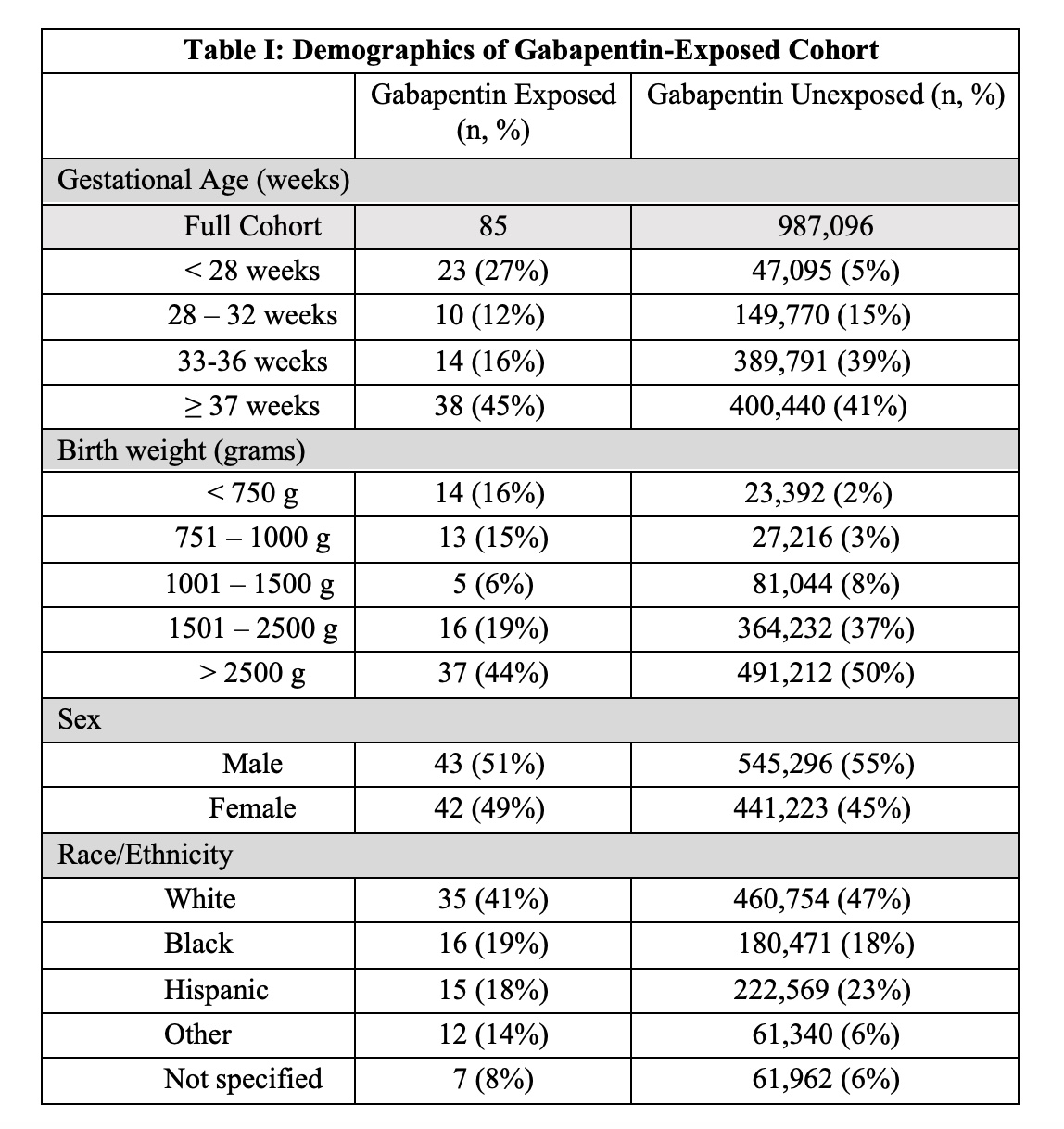
Design/Methods: This is a cohort study of infants born ≥ 22 weeks gestation, exposed to gabapentin, and discharged from NICUs managed by the Pediatrix Medical Group from 2005 to 2020. Frequencies and percentages were measured for categorical variables and interquartile ranges for continuous variables, and data were stratified by gestational age and birth weight (BW). We compared demographic and clinical characteristics differences between infants exposed and not exposed to gabapentin using Chi-square or Fisher exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
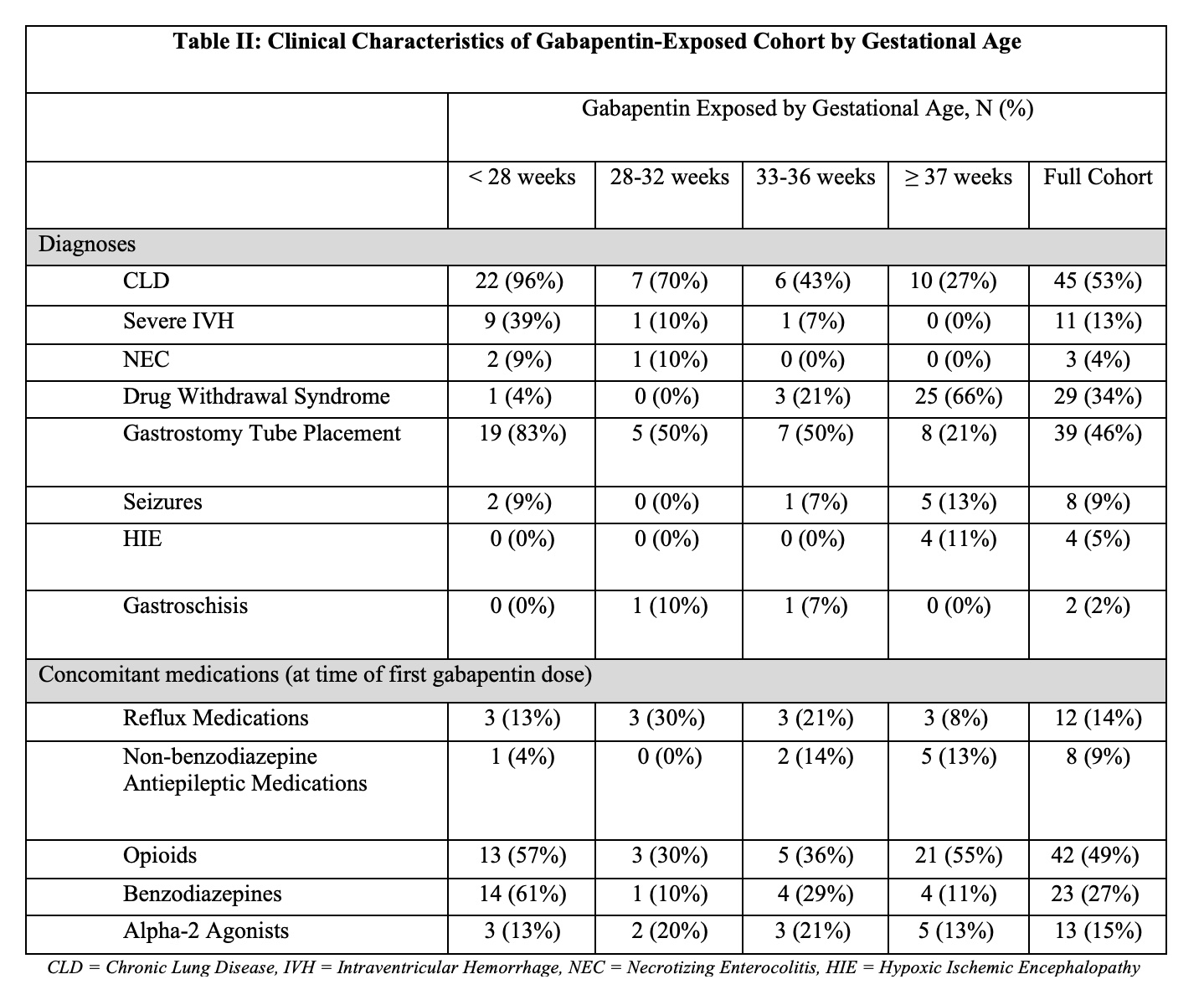
Results: 85 infants received gabapentin from 2005 to 2020. In 2016, 0.012% of infants received gabapentin compared to 0.036% in 2020, an increase of 0.024% (p < 0.01). The median treatment length was 39 days (25th, 75th percentiles: 7, 64). The median BW of gabapentin-exposed infants compared to unexposed infants was 2200g (876g, 3080g) and 2500 g (1890g, 3210g) respectively (p < 0.001). Over half (59%) of infants who received gabapentin were born prematurely ( < 37 weeks gestation). Length of stay ranged from a median 94 days (51, 169) compared to 10 days (6, 22) in the unexposed group (p < 0.001). 53% (n=45) who received gabapentin had chronic lung disease (CLD), 46% (n=39) received gastronomy tubes, and 34% (n=29) had drug withdrawal syndrome (DWS). Only 9% (n=8) had seizures. 49% (n=42) received opioids and 27% (n=23) received benzodiazepines.
Conclusion(s): Gabapentin use increased in our cohort over the studied time period, including a three-fold increase from 2016 to 2020. Gabapentin was tied to lower BW, prematurity, and a longer hospital stay. Gabapentin-exposed infants were likely to receive opioids and benzodiazepines and have CLD, gastrostomy tubes, and DWS. More studies are needed to determine the safety, efficacy, and proper dosing of gabapentin in infants.
.jpg)