Neonatology
Session: Neonatal General 5: PDA, Surgical Conditions, Infections
142 - Standard versus High-Dose Oral Ibuprofen for the Treatment of Persistent Patent Ductus Arteriosus in Extremely Low Gestational Age Neonates: Exposure-Response relationship in Target Population.
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 142
Publication Number: 142.2237
Publication Number: 142.2237
- VS
Vivian Slade, BSc, PharmD, RPh, ACPR (she/her/hers)
McMaster University Michael G. DeGroote School of Medicine
Hamilton, Ontario, Canada
Presenting Author(s)
Background: Oral ibuprofen is the preferred pharmacotherapeutic option for the treatment of persistent patent ductus arteriosus (PDA) for its favorable efficacy-safety profile. This data, however, mainly originates from pharmacokinetic (PK) and pharmacodynamic (PD) evidence on ibuprofen’s intravenous (IV) formulation and in neonates of higher gestational age (GA) as compared to the current target population of extremely low gestational age neonates (ELGANs).
Objective: To describe the degree of exposure to oral ibuprofen (area under the concentration-time curve [AUC]) in ELGANs receiving standard versus high-dose oral ibuprofen treatment for closure of a persistent PDA and its association with the occurrence of serious Adverse Events (AEs).
Design/Methods: This was a retrospective cohort study of neonates with gestational age of ≤ 28+6 weeks on standard (10, 5, 5 mg/kg in postnatal age (PNA) ≤ 72hours) or high (20, 10, 10 mg/kg in PNA > 72 hours) dosing regimen of oral ibuprofen for closure of a persistent PDA. Exposure at 24 (AUC0-24) and 72 (AUC0-72) hours, rate of closure of a persistent PDA, need for a repeat pharmacotherapy, surgical ligation, the occurrence of serious AEs and mortality were examined in both groups.
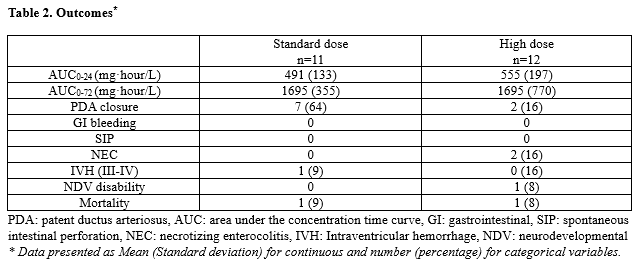
Results: Standard and high dose of oral ibuprofen were administered to 12 and 11 neonates, respectively. There were no differences in the baseline and the echocardiographic characteristics of the PDA across the two groups (Table 1). In the standard and high treatment groups, the rate of exposure (AUC0-24 and AUC 0-72 (mg·hour/L) - Mean, SD) was 491 (133) and 555 (197) at 24 hours (AUC0-24) and 1695 (770) and 1695 (355) at 72 hours (AUC0-72), respectively (p > 0.05). The number of neonates with successful closure of PDA was 7 (63%) and 2 (17%) in the standard and high treatment groups (p < 0.05). Three serious AEs were identified (2 in high and 1 in standard dosing groups). The rate of exposure to ibuprofen was not different in neonates with occurrences of serious AEs as compared to the rest of patients. Upon utilizing the casualty assessment algorithm by Due et. al, it was determined that the likelihood of the three serious adverse events being the presumed result of ibuprofen was low by two independent reviewers (MR and SSZ).
Conclusion(s): In ELGANs with a persistent PDA, the use of standard and high-dose oral ibuprofen for neonates with PNA of < 72 hours and those > 72 hours, respectively, results in comparable exposure. Future studies on the PNA-dependent changes of PD response to oral ibuprofen and the AE profile of this drug among ELGANS with persistent PDA are needed.
.png)