Neonatology
Session: Neonatal-Perinatal Health Care Delivery: Practices and Procedures 3
474 - Association between Medication for Opioid Use Disorder During Pregnancy on Neonatal Outcomes
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 474
Publication Number: 474.3048
Publication Number: 474.3048

Richard W. Hall, MD (he/him/his)
Professor
University of Arkansas for Medical Sciences College of Medicine
Little Rock, Arkansas, United States
Presenting Author(s)
Background: Medication for Opioid Use Disorder (MOUD) for prenatal opioid exposure is currently recommended for pregnancies affected by opioid dependency. Studies comparing opioid agonists for MOUD have suggested buprenorphine may be preferable to methadone but there are neonatal concerns for both medications. There are limited data evaluating the association between MOUD exposure and neonatal outcomes.
Objective: We investigated the association between: 1) MOUD exposure and neonatal outcomes in neonates with neonatal opioid withdrawal syndrome (NOWS); and 2) MOUD type (buprenorphine only vs. methadone only) and neonatal outcomes in neonates with NOWS exposed to MOUD.
Design/Methods: Data were collected from medical records of pregnant women and their neonates with NOWS from 13 hospitals enrolled in the Informing NOWS Research through Retrospective Data Collection study. This analysis included neonates exposed to antenatal opioids during the second and/or third trimesters of pregnancy and who were 36 to 41 weeks gestational age at birth. MOUD was defined as neonates exposed to prescribed MOUD. Non-MOUD was defined as neonates exposed to illicit opioids, prescribed opioids for pain, or non-prescribed buprenorphine or methadone.
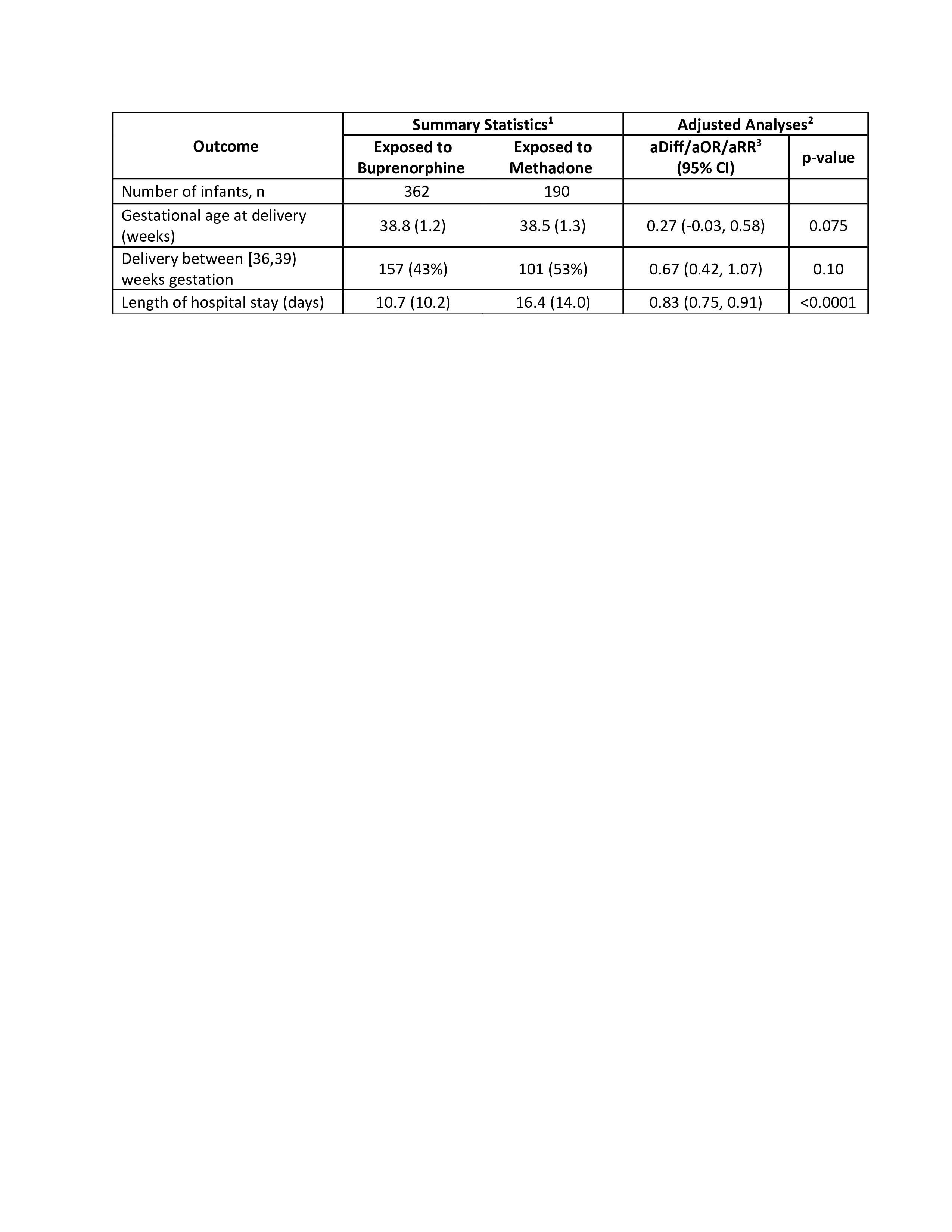
Results: Of the 765 neonates with NOWS included in this study, 573 (75%) were exposed to MOUD and 192 (25%) were exposed to non-MOUD opioids. Of the neonates with NOWS exposed to MOUD, 362 (63.2%) were exposed to buprenorphine, 190 (33.2%) were exposed to methadone, and 21 (3.7%) were exposed to a combination or some other medication. Compared to non-MOUD neonates, MOUD neonates experienced a 57% lower odds of delivery before 39 weeks (odds ratio=0.43; 95% CI: (0.31,0.58); p< 0.0001) and a 2.75-fold higher odds of breastfeeding (95% CI:(1.95,3.89); p< 0.0001), with similar length of hospital stay and birthweight and head circumference z-scores. However, MOUD neonates experienced a 1.38% greater percentage of weight loss during hospitalization (95% CI: (0.71,2.05); p< 0.0001) (Table 1). Compared to neonates with NOWS exposed to methadone, neonates with NOWS exposed to buprenorphine experienced a 17% reduction in hospital stay (relative rate=0.83; 95% CI: (0.75,0.91); p< 0.0001) with a trend for a greater percentage of neonates delivering after 39 weeks (Table 2).
Conclusion(s): MOUD is associated with improved neonatal outcomes, including fewer deliveries before 39 weeks and a higher incidence of breastfeeding. Exposure to buprenorphine during pregnancy was associated with a shorter length of hospital stay.
.jpg)