Breastfeeding/Human Milk
Session: Breastfeeding/Human Milk 2: Human Milk Feeding
486 - Intranasal Human Milk as Stem Cell Therapy for Intraventricular Hemorrhage in Preterm Infants: Long-term Outcomes to 18 months
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 486
Publication Number: 486.2008
Publication Number: 486.2008

Rebecca Hoban, MD MPH (she/her/hers)
Staff Neonatologist
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: Intraventricular hemorrhage (IVH) is a common cause of brain injury with neurodevelopmental sequalae for preterm infants. Stem cell (SC) therapies hold promise for brain injury; fresh human milk (HM) contains pluripotent SCs that produce neuronal cells in vitro. Permeable neonatal blood brain barriers potentially allow SC delivery to the brain via fresh intranasal HM (IHM) administration. We performed the first prospective pilot of IHM administration in preterm neonates with IVH with neurodevelopmental follow-up to 18 months.
Objective: To compare neurodevelopmental outcomes of infants with IVH who received IHM to HM-fed infants with IVH from the same NICUs the year prior.
Design/Methods: Recruitment took place at 2 tertiary care NICUs in Toronto. Infants < 33 weeks gestation with IVH with a lactating parent were eligible. IHM was given ideally 2x/day until day 28 of age This safety and feasibility trial was not powered for outcomes. Neurodevelopmental testing and HM feeding history were collected from routine NICU follow-up clinic visits at 4, 8, 12, and 18 months corrected age (CA) and summarized using descriptive statistics. 18 month follow-up is ongoing for some prospective subjects. Detailed case-control analyses are underway to control for high proportions of grade 4 IVH in the prospective cohort.
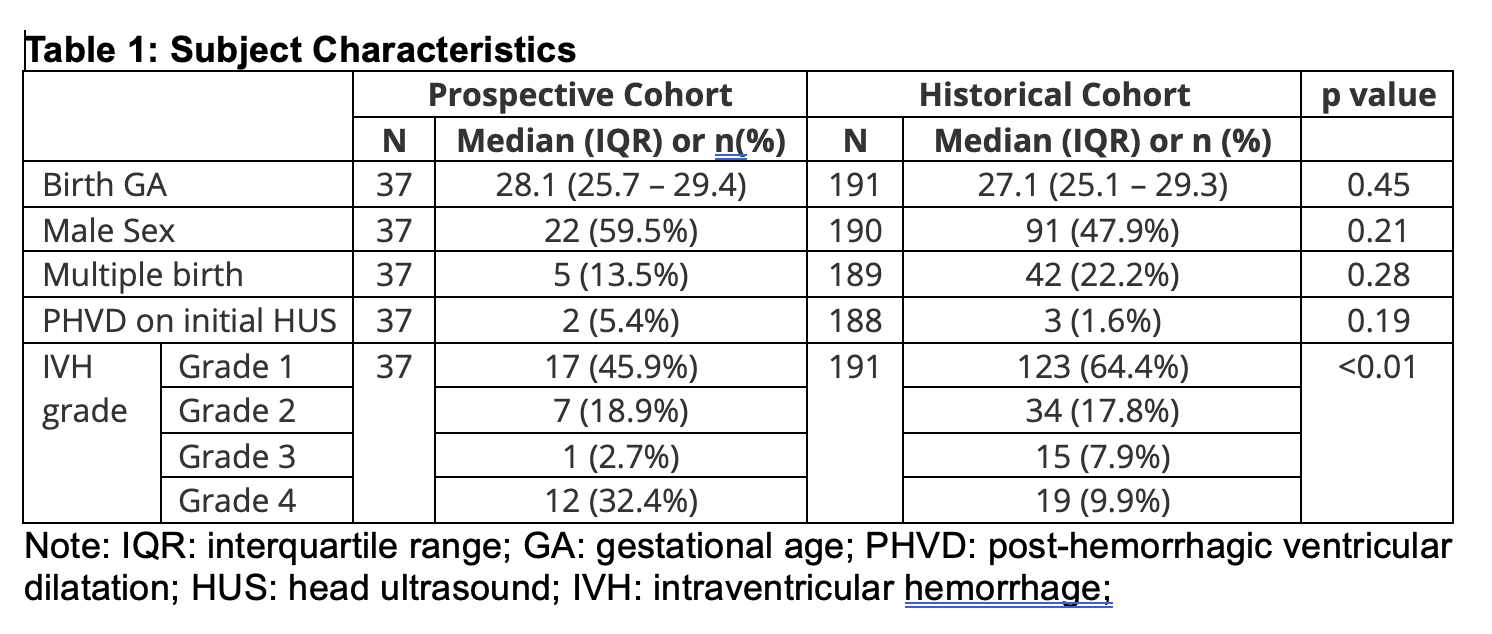
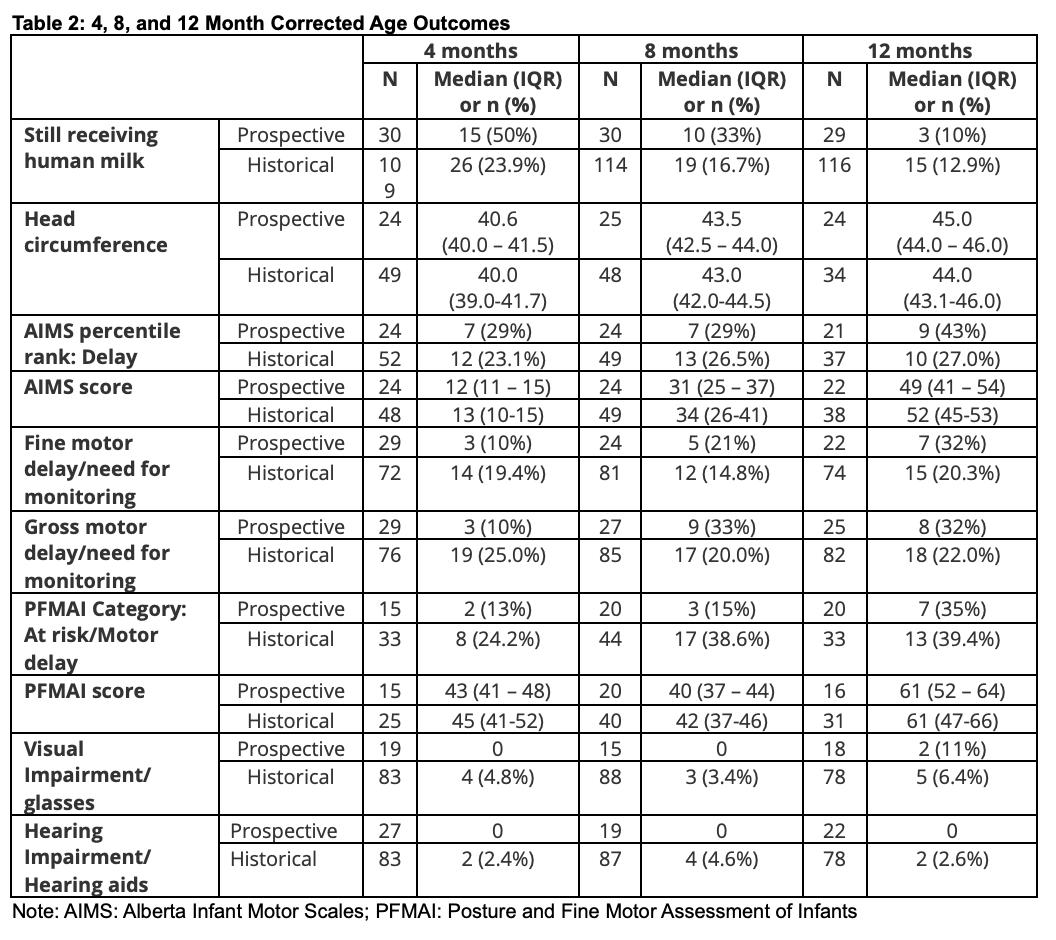
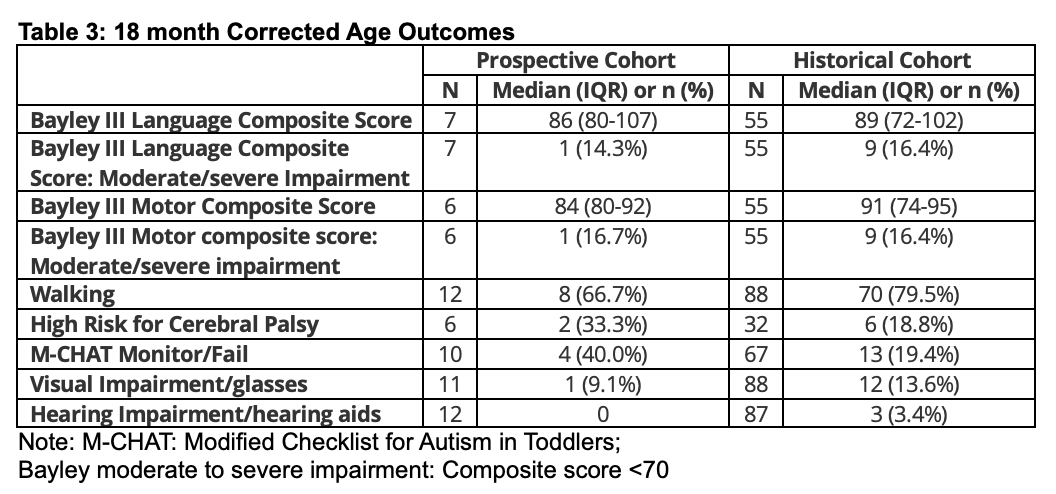
Results: 33/37 preterm infants who received IHM and 171/191 historic controls survived to NICU discharge (Table 1). Trial infants were higher-risk neurodevelopmentally, with higher rates of grade 4 IVH, necrotizing enterocolitis (22.2% vs 14.3%), and retinopathy of prematurity >=Stage 3 (26.3% vs 13.1%). Follow-up rates were suboptimal during the pandemic. Outcomes to 12 months CA (Table 2) appeared similar between groups without yet controlling for differences in severe IVH prevalence between cohorts. HM feeding persisted longer in the prospective cohort. 18 month CA outcomes (Table 3) are being finalized and will be analyzed to control for high grade 4 IVH prevalence in the prospective cohort.
Conclusion(s): In this unpowered pilot study, preterm infants who received IHM had high prevalence of grade 4 IVH, putting them at risk of poor outcome. Analyses controlling for grade of IVH are underway, but overall outcomes to 18 months CA appear similar to historical controls, in which rates of high-grade IVH were much lower. Taking part in the trial was associated with longer HM feeding, suggesting parental education about SCs in HM and involving them in a HM trial may increase lactation duration, which independently improves outcomes.