Neonatology
Session: Neonatal-Perinatal Health Care Delivery: Practices and Procedures 3
475 - Nonpharmacologic Care and the Finnegan Scoring Tool: Have National Recommendations Improved Hospital Outcomes for Infants with NOWS?
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 475
Publication Number: 475.3094
Publication Number: 475.3094

Lori A. Devlin, DO, MHA, MS (she/her/hers)
Professor Pediatrics
University of Louisville School of Medicine
Louisville, Kentucky, United States
Presenting Author(s)
Background: Neonatal Opioid Withdrawal Syndrome (NOWS) is a frequent outcome after antenatal opioid exposure. Optimization of non-pharmacologic treatment for infants with NOWS is associated with improved outcomes and endorsed in the 2020 American Academy of Pediatrics (AAP) recommendations. Nonpharmacologic care (NPC) as initial treatment, improves outcomes for infants with NOWS managed with the Eat, Sleep, Console care approach, but it needs to be determined if enhanced NPC improves hospital outcomes for infants assessed with the Finnegan Neonatal Abstinence Scoring Tool (FNAST).
Objective: To examine differences in initiation and duration of opioid treatment for NOWS, pre-and post- publication of national recommendations to optimize NPC (the studied epoch), in infants with NOWS assessed with the FNAST.
Design/Methods: INFORM NOW: Informing NOWS Research through Retrospective Data Collection is a multicenter observational cohort study of infants with antenatal opioid exposure from 13 US hospitals. Data were abstracted from the medical records of pregnant women and their infants who were greater than or equal to 36 weeks’ gestation and exposed to opioids during the second and/or third trimesters of pregnancy and divided in pre-epoch (10/01/2019–12/31/2019) and post-epoch (07/01/2021–03/31/2022) time periods. Analyses were performed using generalized estimating equations.
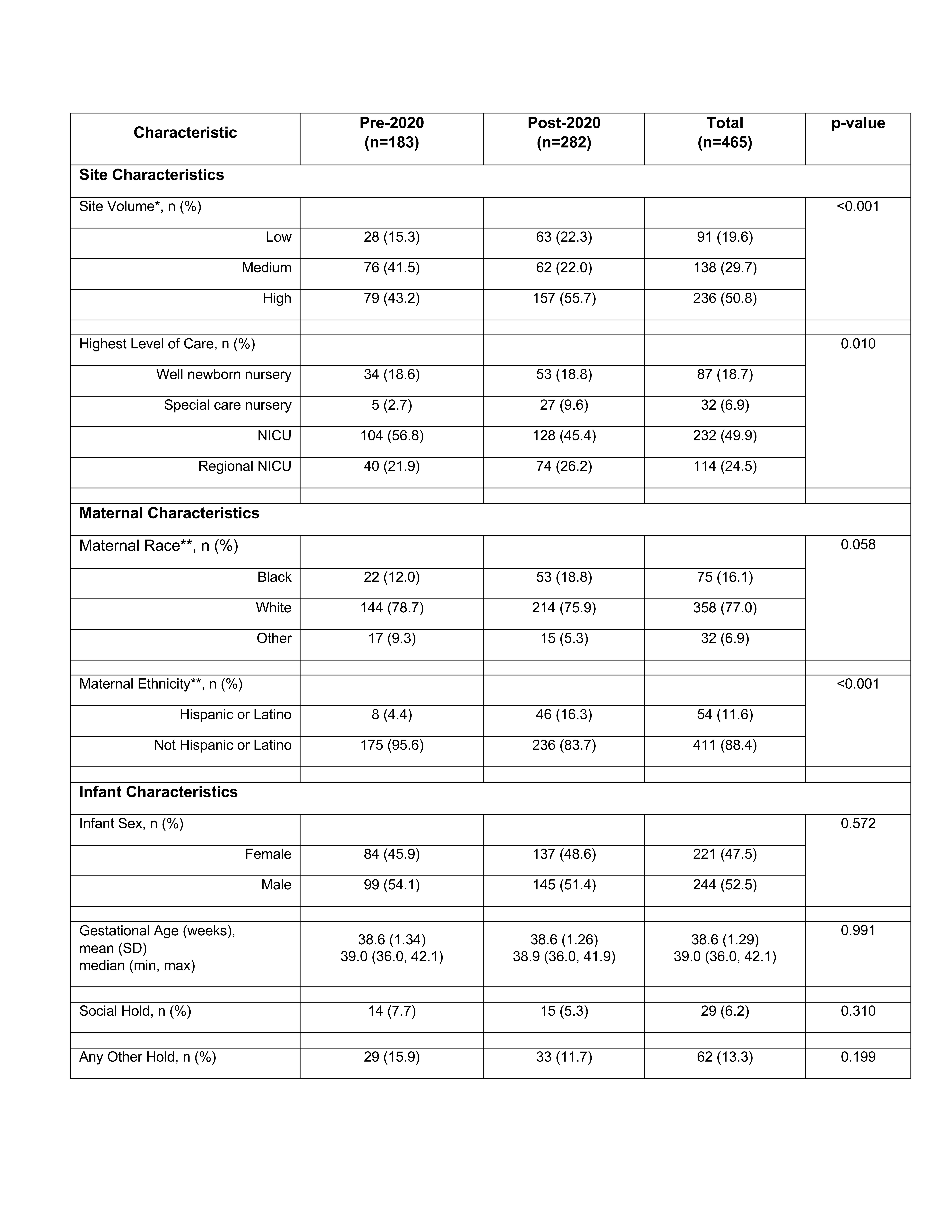
Results: The study included 465 infants assessed with the FNAST; pre-epoch n=183 and post-epoch n=282. Demographic data are presented in Table 1.
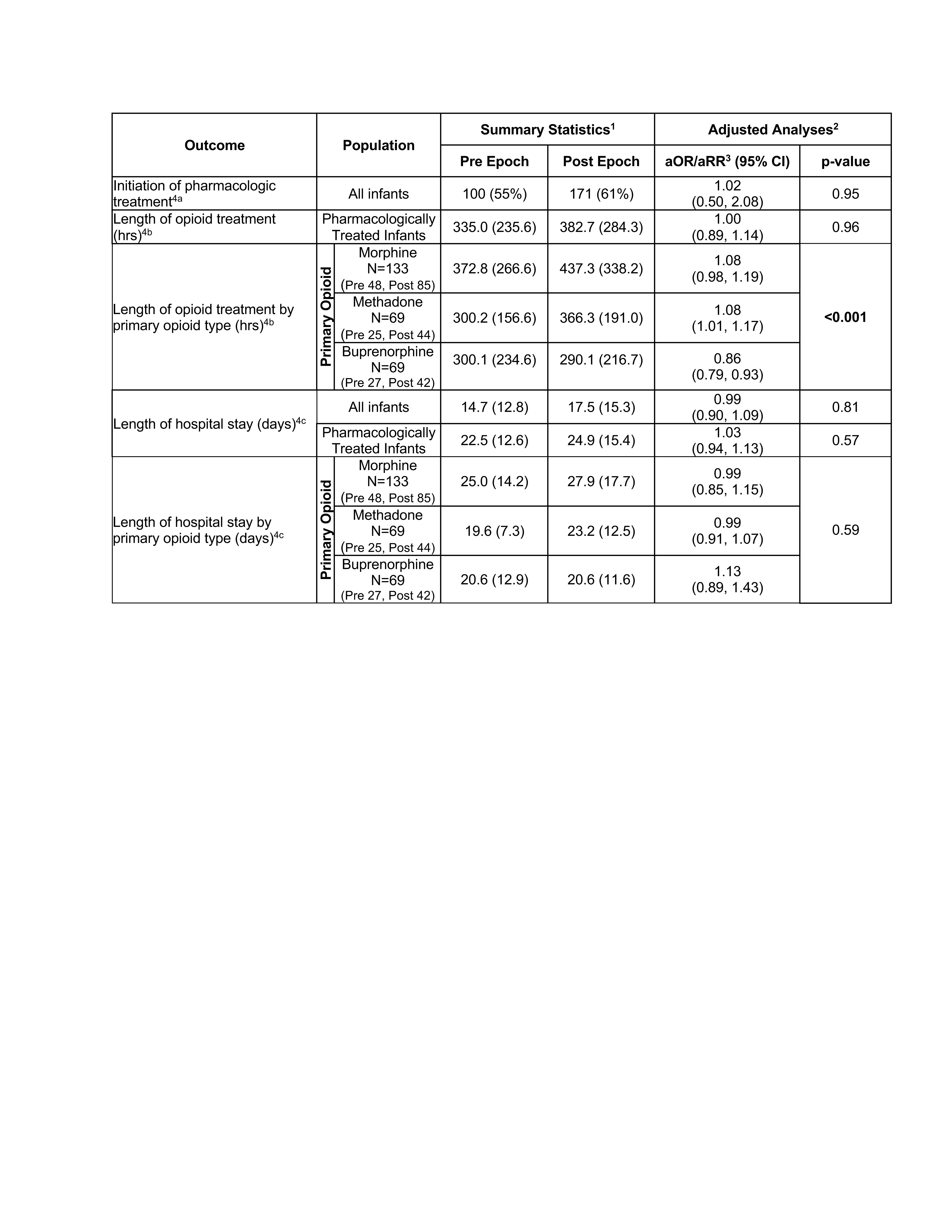
No differences were noted between epochs for initiation and length of pharmacologic treatment (LOT), and length of stay (LOS) for all infants or for infants who were pharmacologically treated. Among infants who received pharmacologic treatment, those treated with buprenorphine had a 14% shorter LOT in the post- vs pre-epoch (aRR=0.86; 95% CI:0.79,0.93) and those treated with methadone had an 8% longer LOT in the post-vs pre-epoch (aRR=1.08; 95%CI:1.01,1.17). Primary opioid type did not modify the relationship between epoch and LOS. (Table 2)
Conclusion(s): The 2020 AAP recommendations for enhanced NPC were overall not associated with a change in the initiation or length of pharmacologic treatment for infants with NOWS assessed with the FNAST. Further studies are required to determine if buprenorphine therapy for NOWS shortens duration of therapy compared to morphine.