Neonatology
Session: Neonatal Clinical Trials 1
471 - Development of the Better Research Interactions for Every Family (BRIEF) Intervention to support recruitment for neonatal clinical trials: an intervention mapping guided approach
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 471
Publication Number: 471.1581
Publication Number: 471.1581

Elliott M. Weiss, MD, MSME (he/him/his)
Associate Professor
Seattle Children's
Seattle, Washington, United States
Presenting Author(s)
Background: Recruitment for neonatal clinical trials is particularly challenging due to the highly vulnerable population, short enrollment windows, and enrollment during a time of high parent stress. The best available evidence suggests disparities in enrollment for marginalized and minoritized populations. This diminishes data quality, leads to less generalizable results, and ultimately threatens to exacerbate existing healthcare disparities.
Objective: We aimed to develop a research team member-facing educational intervention to improve the approach for recruitment for neonatal clinical trials. The Better Research Interactions for Every Family (BRIEF) had two stated goals: 1) improve the experience of recruitment for parents, and 2) increase trial participation, with a focus on decreasing disparities in research participation.
Design/Methods: We chose Intervention Mapping (IM) to guide all steps in developing, implementing, and assessing the intervention. IM is a planning method designed to enable researchers to move from theory to intervention systematically and rigorously by explicitly mapping the behavioral determinants to an intervention’s characteristics, goals and evaluation.
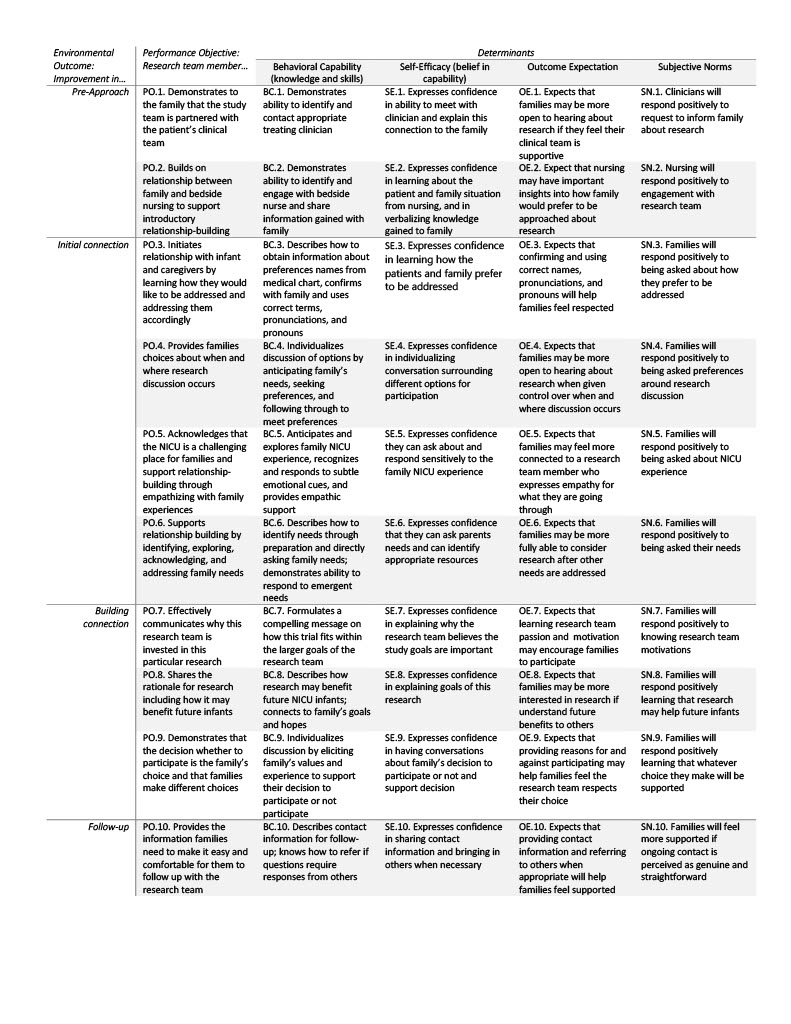
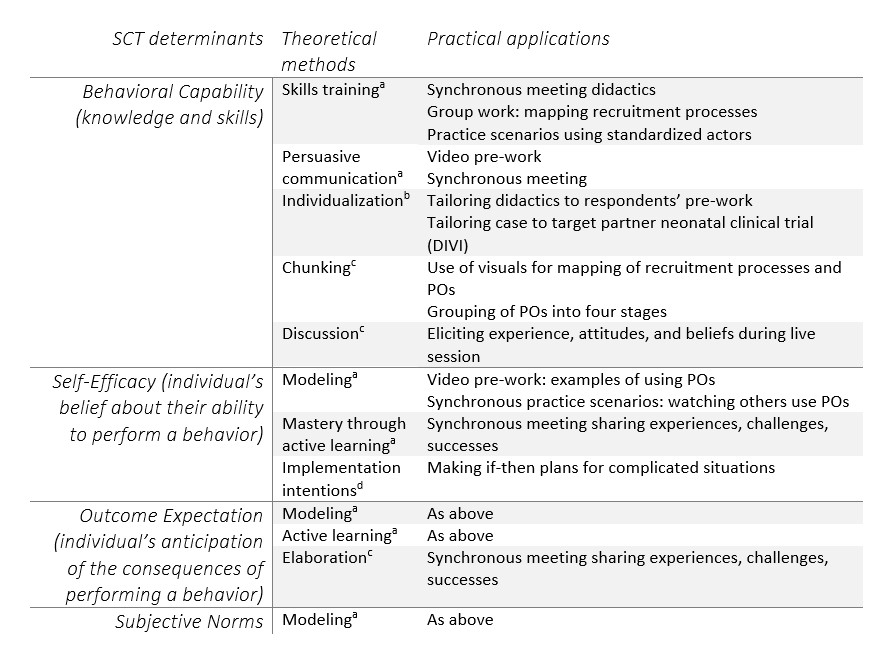
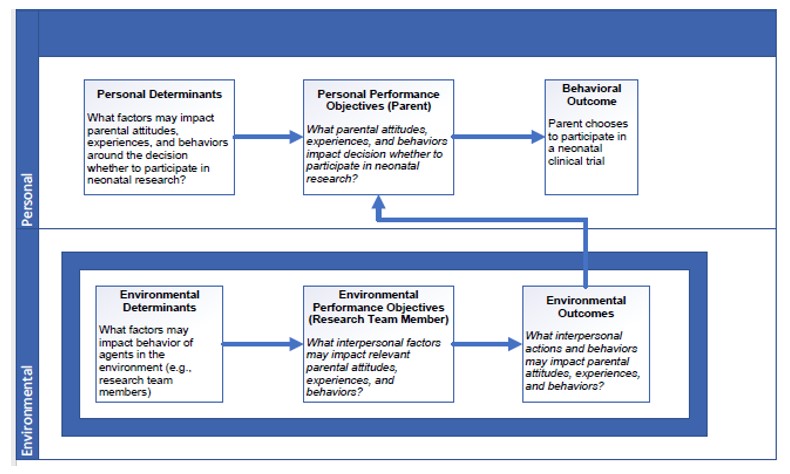
Results: Following the six steps of IM, we: 1) performed a needs assessment through literature as well as our own empirical survey and interview work, resulting in a logic model of change (Figure); 2) created matrices to determine goal changes in behavior by research team members and methods through which this change would occur (see Table 1); 3) selected theoretical methods and practical strategies for the intervention; 4) designed the intervention components (see Table 2); 5) created an implementation plan; and 6) created an evaluation plan, which includes both proximal outcomes (i.e., researcher self-assessment, parent assessment of interaction) and distal outcomes (i.e., enrollment rates). Pilot testing of the BRIEF Intervention within a neonatal clinical trial is underway.
Conclusion(s): This project details the first recruitment intervention for neonatal clinical trials utilizing IM, which supports evidence and theory-based intervention development and intermediate outcomes for intervention refinement. Targeting research team members is a strength of our approach, given the huge variability in their roles, training, experience, and approach to recruitment. After completion of pilot testing, we will refine the BRIEF Intervention and prospectively test it within a multi-site neonatal clinical trial.