Neonatology
Session: Neonatology Pulmonology Clinical Science 2 - Lung Development, Inflammation, Intrauterine environment
590 - Fetal inflammation induces long-term memory to subsequent pulmonary inflammation
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 590
Publication Number: 590.1269
Publication Number: 590.1269
- DA
Deepthi Alapati, MD
Associate Professor of Pediatrics
Nemours Children's Hospital
Wilmington, Delaware, United States
Presenting Author(s)
Background: Fetal inflammation caused by chorioamnionitis, the most frequent identifiable cause of preterm births, causes poor long-term respiratory health. However, the impact of fetal inflammation on pulmonary epithelial cells and response to second hit injury in adulthood is unknown. We hypothesized that a single trigger of inflammation during late gestation in fetal mice will induce altered response to a second hit-inflammatory injury in adulthood.
Objective: To analyze differential gene expression in pulmonary epithelial cells in response to in-utero lipopolysaccharide (LPS)- induced fetal inflammation and responses to pulmonary inflammation in adult mice exposed to LPS- induced chorioamnionitis
Design/Methods: Pregnant Sprague-Dawley rats were injected with intra-amniotic (IA) LPS or normal saline (NS) at E20. Lungs were harvested on postnatal day 1 and Epcam+ epithelial cells were sorted using flow cytometry and subjected to RNA sequencing. Differentially expressed genes with two-fold change and false discovery rate (FDR) < 0.05 were used for gene ontology analysis using DAVID software. To analyze response to second injury in adult mice, pregnant B6N.Cg-Tg(Sftpc,-EGFP)1Dobb/J transgenic mice containing surfactant protein C driving expression of EGFP to identify alveolar epithelial (AT2) cells were injected with IA LPS or NS at E16. At one month of age, LPS or NS was instilled intra-pharyngeal (IP) to induce pulmonary inflammation and lungs were harvested 24 hours later for flow cytometric analysis. Statistical analysis was performed with one-way ANOVA followed by Tukeys multiple-comparison.
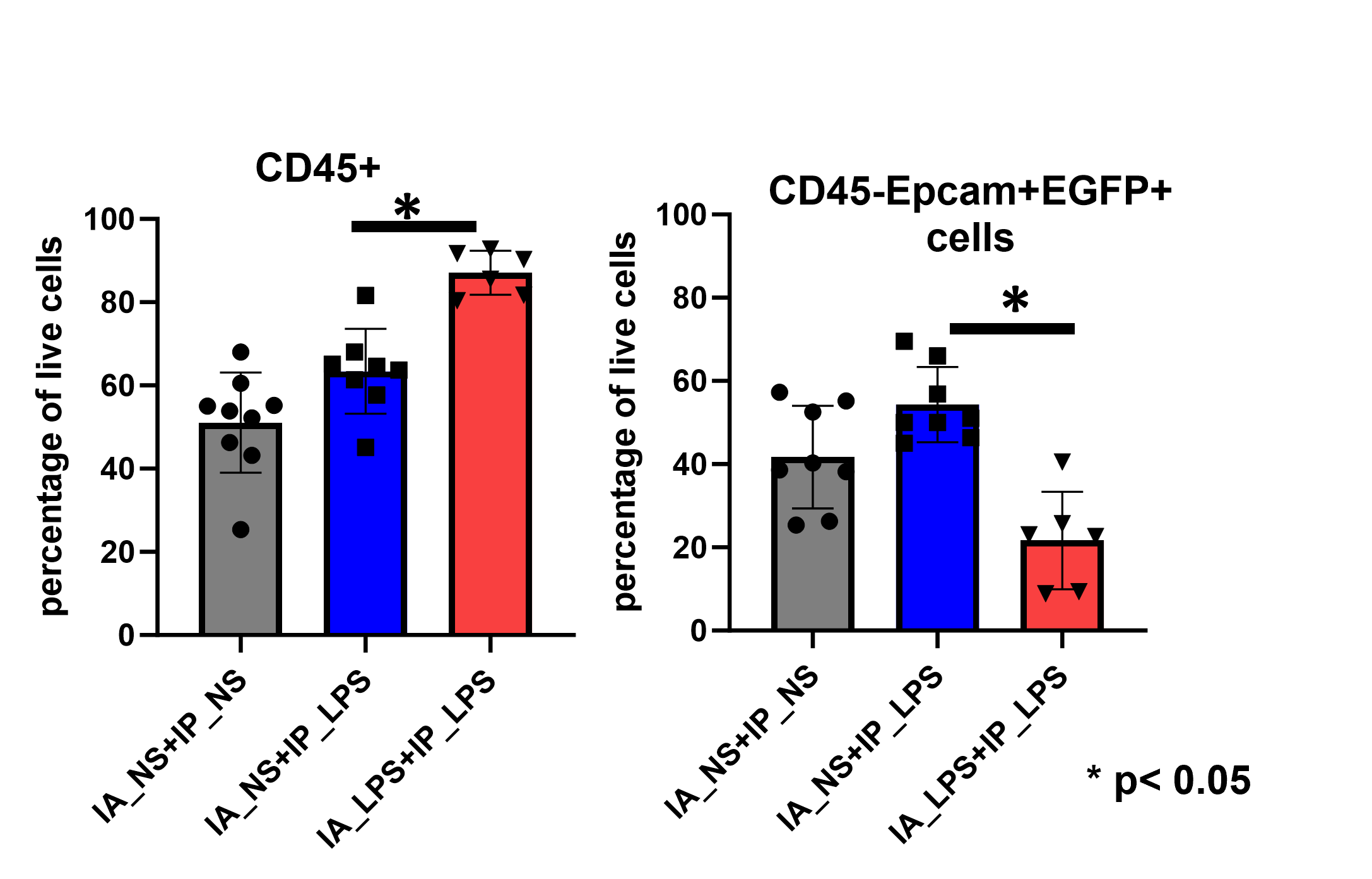
Results: At P1, IA LPS induced expression of pro-inflammatory cytokines, upregulation of genes involved in cell adhesion and angiogenesis and downregulation of genes involved in intermediate filament organization and epithelial cell differentiation. Second-hit injury with IP LPS at 1month of age resulted in greater weight loss in mice exposed to IA LPS compared to mice exposed to IA NS (Fig 1). Mice exposed to fetal inflammation had significantly higher percentage of CD45+ pulmonary cells and significantly lower percentage of GFP labelled AT2 cells compared to controls 24 hours post second-hit inflammation with IP LPS (Fig 2).
Conclusion(s): This study suggests that a single exposure to LPS-induced fetal inflammation results in altered transcriptome signature in pulmonary epithelial cells and long-term memory to subsequent pulmonary inflammation in adulthood.
.png)