Neonatology
Session: Neonatal Clinical Trials 1
466 - Early hydrocortisone for BPD prevention: meta-analysis and effect size modifiers
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 466
Publication Number: 466.1387
Publication Number: 466.1387

Daniele De luca, MD, PhD (he/him/his)
Full Professor of Neonatology
Paris Saclay University
Clamart, Ile-de-France, France
Presenting Author(s)
Background: Hydrocortisone was shown to confer a better benefit/risk balance over dexamethasone which was advantageous in terms of BPD but detrimental for the neurodevelopment. It is likely that the effect of hydrocortisone would vary by patient characteristics. Patient subgroups should be identified based on their clinical characteristics to personalize the treatment and optimize the benefits. Therefore, we performed a systematic review and meta-analysis of randomized controlled trials (RCT) of systemic hydrocortisone, started before the 15th day of life, in very preterm neonates and we investigated the possible effect size modifiers.
Objective: To clarify if systemic hydrocortisone, started before the 15th day of life, in very preterm neonates prevents bronchopulmonary dysplasia (BPD) or other adverse outcomes of prematurity and identify possible effect size modifiers.
Design/Methods: Systematic review and meta-analysis following PRISMA guidelines including multiple meta-regressions to in defy possible effect size modifiers and patient subgroups of interest. Finally , a review of biological plausibility will be added to back the results.
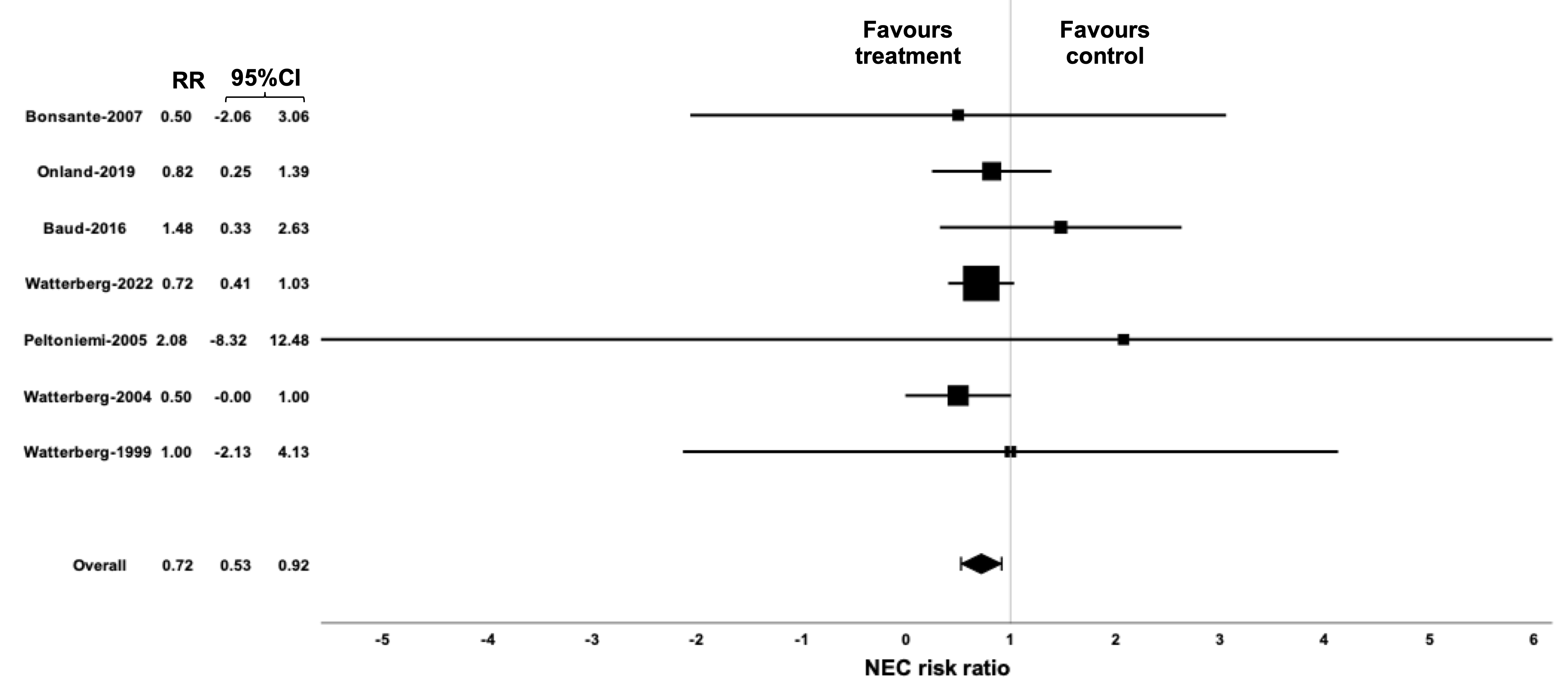
Results: Seven trials were included, they were of general good quality and accounted for a total of 2193 infants. Hydrocortisone treatment did not reduce BPD (RR: 0.84 (95%CI: 0.64-1.04)) although heterogeneity was evident (I2=51.6%). The effect size for BPD is greatest for 10-12 days duration of treatment (= 0.032 (0.01); p=0.007) and tended to be greater in patients with chorioamnionitis (= -1.5 (0.841); p=0.07). Hydrocortisone treatment may significantly reduce mortality (RR: 0.75 (95%CI: 0.59-0.91)), there is no heterogeneity (I2=0) and the reduction tended to be greater in males (= -0.06 (0.03), p=0.07). Hydrocortisone may significantly reduce necrotizing enterocolitis (NEC; RR: 0.72 (95%CI: 0.53-0.92)); there is neither heterogeneity (I2=0%), nor any effect size modifiers. Hydrocortisone did not affect other adverse outcomes of prematurity.
Conclusion(s): A meta-analysis of seven trials and 2193 neonates shows that hydrocortisone did not reduce BPD. Hydrocortisone show benefits on some secondary outcomes, that is mortality (particularly in male infants) and NEC, thus it can be considered, on a case-by-case evaluation, for these purposes. There are some potential effect size modifiers for mortality and BPD which should be addressed in future explanatory trials.
.png)