Neonatology
Session: Neonatal GI Physiology & NEC 3: GI Physiology and Probiotics
536 - Association of probiotic use in premature infants and the incidence of necrotizing enterocolitis: a retrospective cohort study
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 536
Publication Number: 536.2011
Publication Number: 536.2011

Vivian Jongejans (she/her/hers)
Post thesis guest employment
Emma Children's Hospital Amsterdam UMC
Amsterdam, Noord-Holland, Netherlands
Presenting Author(s)
Background: Necrotizing enterocolitis (NEC) is a severe inflammatory disease of the intestine predominantly affecting very preterm infants. The pathophysiology of NEC is partly unknown, but appears to arise from multiple risk factors. Dysbiosis of the intestinal microbiome combined with an exaggerated immune response in the preterm gut seem to be the most crucial factors. Randomized controlled trials have shown that, compared with placebo, certain probiotics containing singular- or multiple strains of living bacteria are effective in restoring the altered microbiome in preterm infants, leading to reduced risks in NEC, sepsis and mortality rates.
Objective: To investigate whether the implementation of postnatal administration of probiotics reduced the incidence of NEC ≥stage II in infants born < 30 weeks’ gestation in a non-randomized real life setting.
Design/Methods: We conducted a single center retrospective cohort study analyzing the incidence of NEC ≥stage II in infants born < 30 weeks’ gestation between 2018 and June 2023. We compared an epoch without probiotics (until March 2021) to an epoch with infants treated with routine probiotics. The probiotic product (Proprems®) contained 10^9 CFU Bifidobacterium infantis Bb-02, Bifidobacterium lactis Bb-12, and Streptococcus thermophilus TH-4. Our primary outcome was NEC incidence ≥ stage II. The secondary outcomes were culture-proven sepsis and mortality during admission. Multivariate logistic regression analyses were performed, adjusting for gestational age (GA) and small for gestational age (SGA).
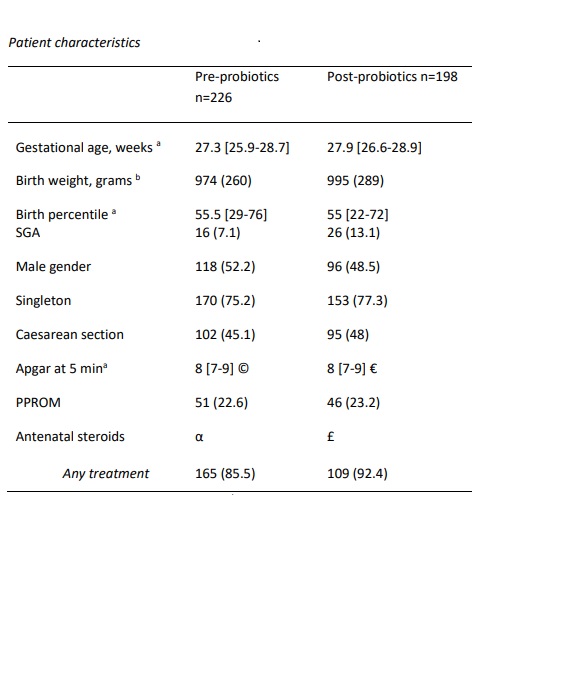
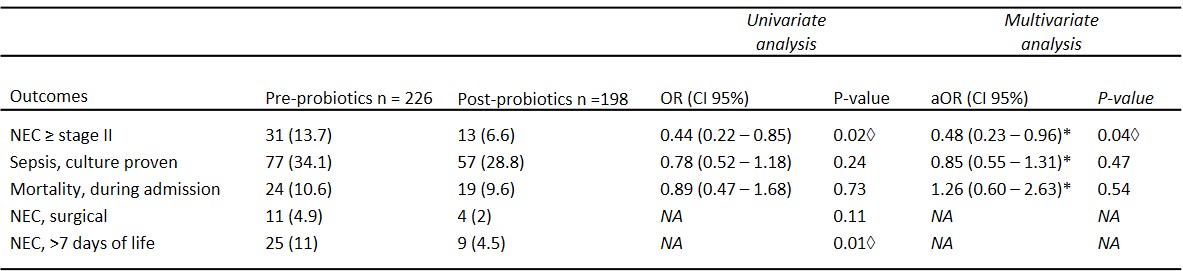
Results: Of the 424 included infants, 226 (53%) infants were admitted in the pre-probiotics epoch, and 198 (47%) in the post-probiotic epoch. There was an increased number of infants SGA in the post-probiotics epoch compared to the pre-probiotics epoch (13.1% vs. 7.1%). There were no differences in other patient baseline characteristics between the two study periods (Table 1). NEC stage ≥II was significantly reduced after probiotics implementation (13.6% vs 6.7%, aOR 0.48, CI 95% [0.23 – 0.96] p=0.04). There was no significant reduction in culture-proven sepsis (34.1% vs 28.8%, aOR 0.85, CI 95% [0.55 – 1.31], p=0.47), or mortality (10.6% vs 9.6%, aOR 1.26, CI 95% [0.60 – 2.63], p=0.54). In the post-probiotics epoch the number of surgical NEC cases was reduced by over 50%, however not significantly (4.9 vs 2%, p=0.11), while the infants developing NEC ≥stage II >7 days of life was significantly reduced by 60% (11% vs 4.5%, p=0.01).
Conclusion(s): Supplementation of selected probiotic strains in infants born < 30 weeks gestation significantly reduced NEC ≥stage II incidence.