Session: Neonatal Neurology 1: Clinical
16 - On Target Dosing: Erythropoietin Exposure in Neonates with Hypoxic-Ischemic Encephalopathy in the HEAL Randomized Controlled Trial
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 16
Publication Number: 16.836
Publication Number: 16.836
Adam Frymoyer, Stanford University School of Medicine, Palo Alto, CA, United States; Ana Gabriela Vasconcelos, University of Washington, Seattle, WA, United States; Sandra E. Juul, University of Washington, Seattle, WA, United States; Bryan A. Comstock, Higher Education, Seattle, WA, United States; Patrick Heagerty, University of Washington, Seattle, WA, United States; Yvonne W. Wu, UCSF, San Francisco, CA, United States

Adam Frymoyer, MD (he/him/his)
Clinical Professor
Stanford University School of Medicine
Palo Alto, California, United States
Presenting Author(s)
Background: A phase 3 randomized, placebo-controlled trial of high-dose erythropoietin (Epo) in neonates with HIE receiving hypothermia (HEAL trial) demonstrated no neurodevelopmental benefit but was associated with a higher rate of serious adverse event (SAE). Selecting the right dose is critical for phase 3 trials. The dosing strategy for HEAL was supported by two early clinical phase safety and pharmacokinetics (PK) studies and targeted Epo exposures associated with neuroprotection in animal models. Understanding if exposure targets were achieved and if SAEs were associated with higher exposures would help future therapeutic programs of Epo as a neuroprotective treatment.
Objective: To evaluate Epo PK in HEAL to determine (a) if target exposures were achieved and (b) whether there was a relationship between higher Epo exposure and risk of SAE.
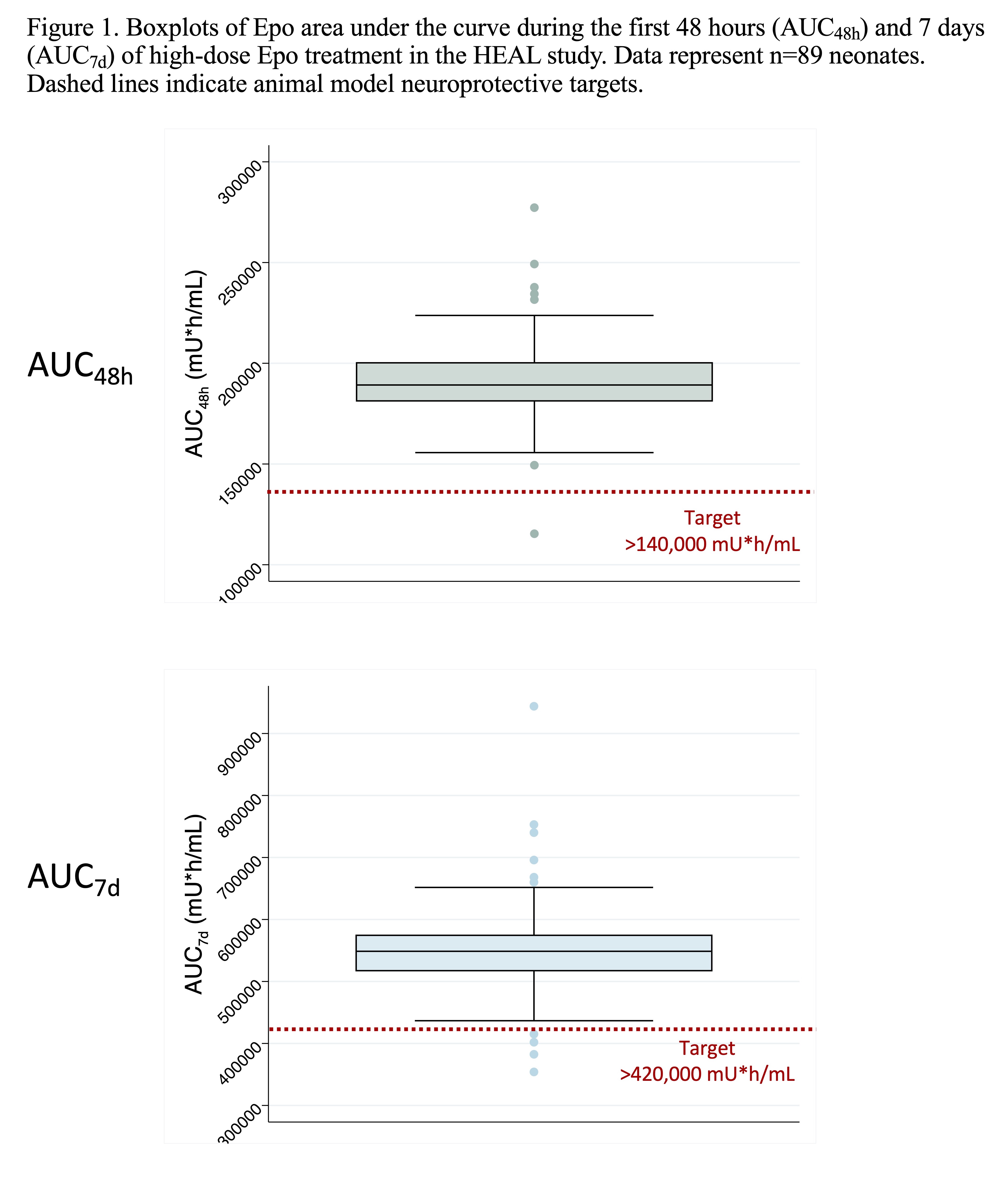
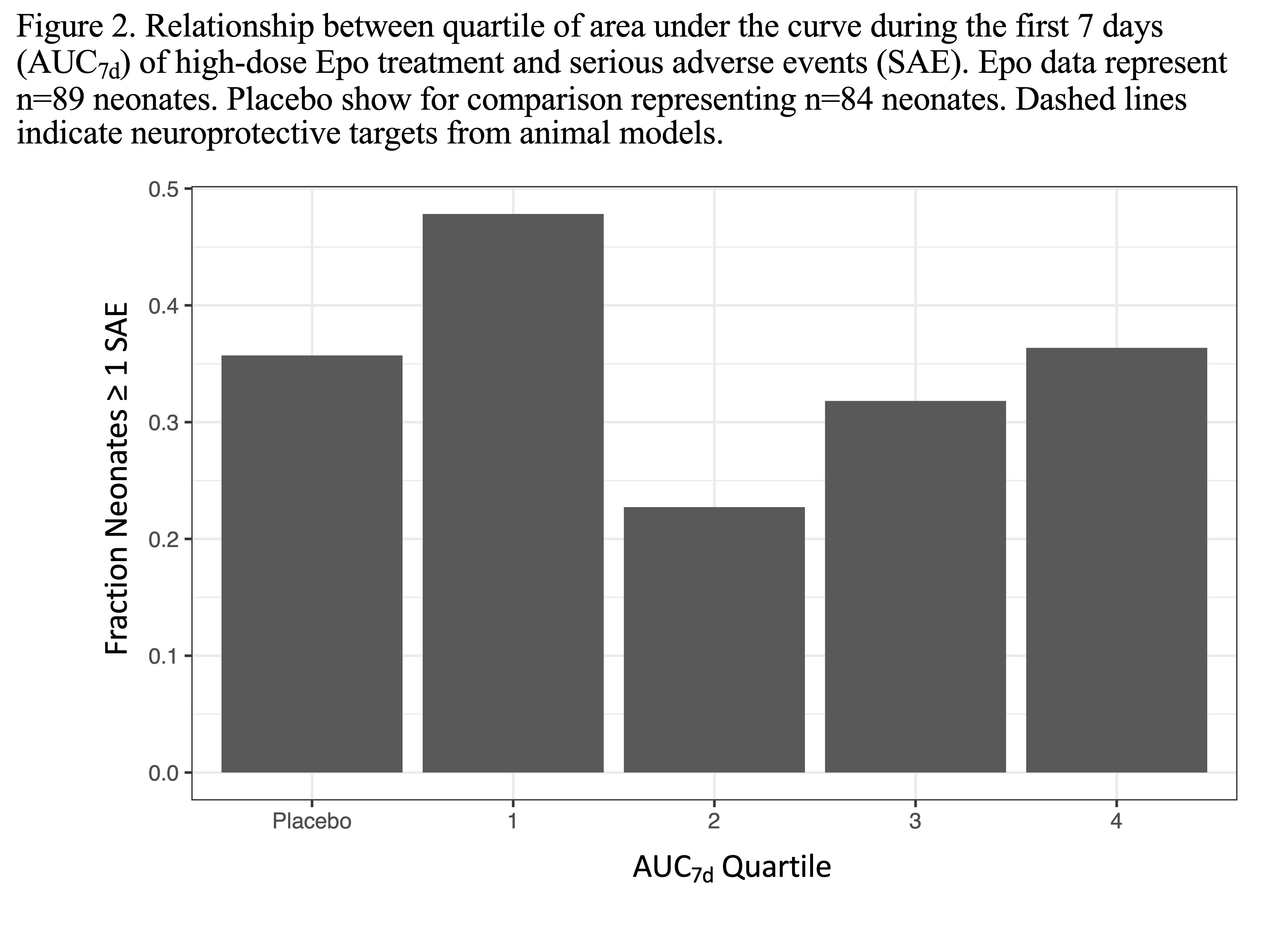
Design/Methods: Secondary analysis of a subset of HEAL subjects who received Epo (1000 U/kg IV on days 1, 2, 3, 4 and 7) and had an Epo plasma concentration measured pre-Epo and on days 2 and 4. A published population PK model of Epo developed in hypothermic neonates with HIE was implemented within a Bayesian framework to predict in each neonate the area under the curve during the first 48 hours (AUC48h) and 7 days (AUC7d) of treatment. The % of neonates who achieved animal model neuroprotective targets of AUC48h >140,000 mU*h/ml and AUC7d >420,000 mU*h/ml were calculated. The relationship between AUC7d and SAE after first study drug dose was also evaluated by logistic regression.
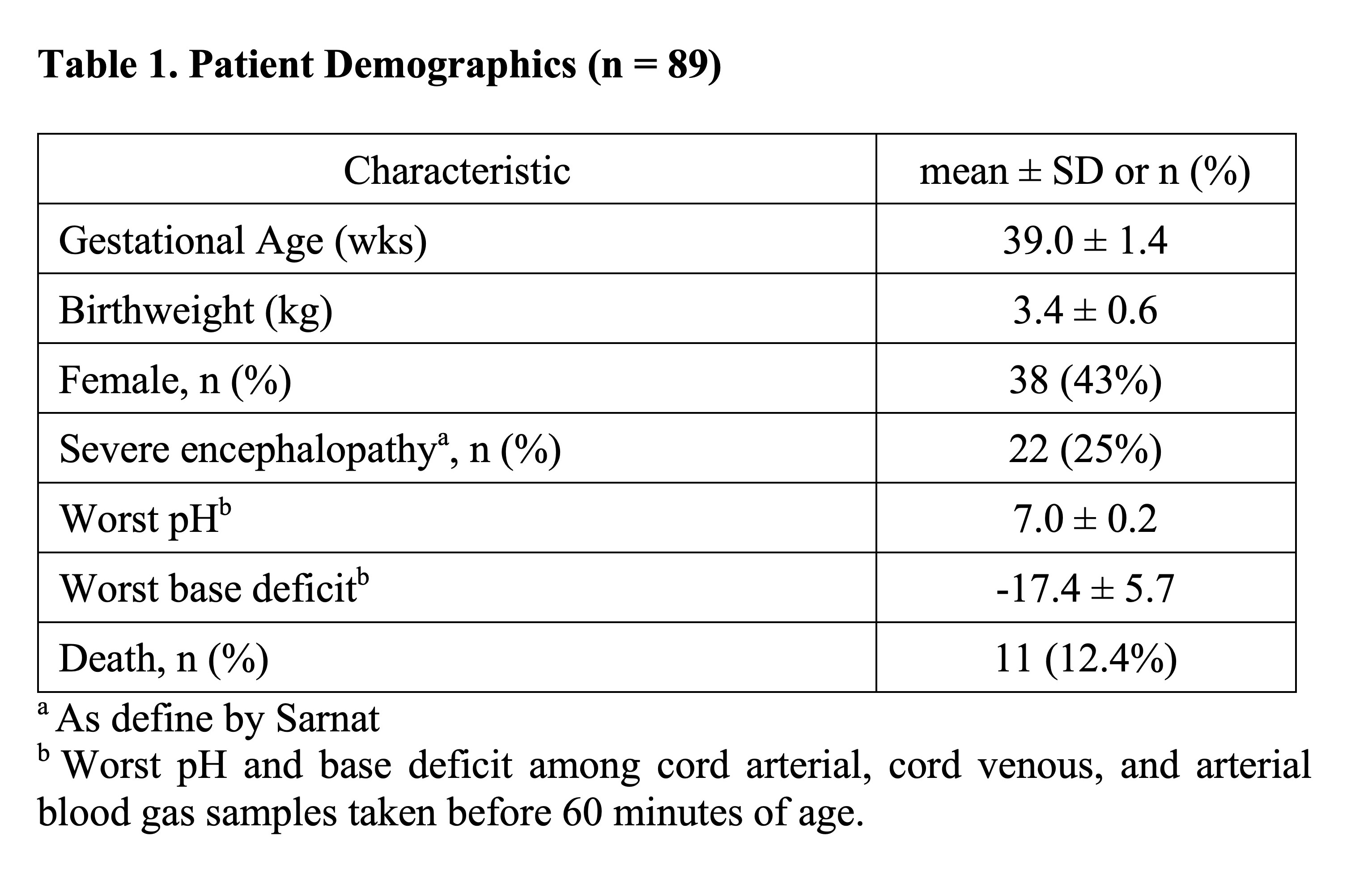
Results: Of 257 neonates randomized to Epo, 89 had plasma concentration data available for analysis (Table 1). The population PK model adequately predicted Epo concentrations in neonates (mean bias -5.4% [95% CI -9.3 to -3.5%]; mean precision 13.1% [95% CI 10.6 to 16.1%]). Variation in Epo exposure between patients was low with AUC48h coefficient of variation 11.2% and AUC7d coefficient of variation 14.1% (Figure 1). Over 98% and 95% of neonates achieved the target AUC48h and AUC7d, respectively. No relationship was seen between AUC7d and risk of SAE (p=0.4; Fig 2).
Conclusion(s): The Epo dosing strategy in the phase 3 HEAL trial consistently achieved target exposures across neonates, and higher exposures were not associated with more frequent SAEs. Early phase safety and PK studies are critical to support dosing strategies in neonatal therapeutic development programs.