Neonatology
Session: Neonatal Quality Improvement 1
387 - Improving Sedation of Mechanically Ventilated Infants in a Free-Standing Children’s Hospital: Evaluating the Impact of a Quality Improvement Intervention after 1 year of implementation.
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 387
Publication Number: 387.2280
Publication Number: 387.2280

Veronica Echanove Iturralde, MD (she/her/hers)
Resident physician
Nicklaus Children’s Hospital
Miami, Florida, United States
Presenting Author(s)
Background: Sedation and analgesia of mechanically ventilated (MV) newborns in the NICU setting creates a delicate task of balancing the neurotoxic and addictive properties of the required medications with the optimization of cardiorespiratory comfort in patients. Current guidelines support a multimodal approach favoring opioids combined with ɑ2 agonists over benzodiazepines.
Objective: In July 2022, we implemented a quality improvement initiative to improve sedation of mechanically ventilated infants. The aim of the project was to increase the percentage of continuous IV sedation days using a multimodal approach favoring administration of opioids and ɑ2 agonists. The secondary outcome was to reduce the overall use of opioids.
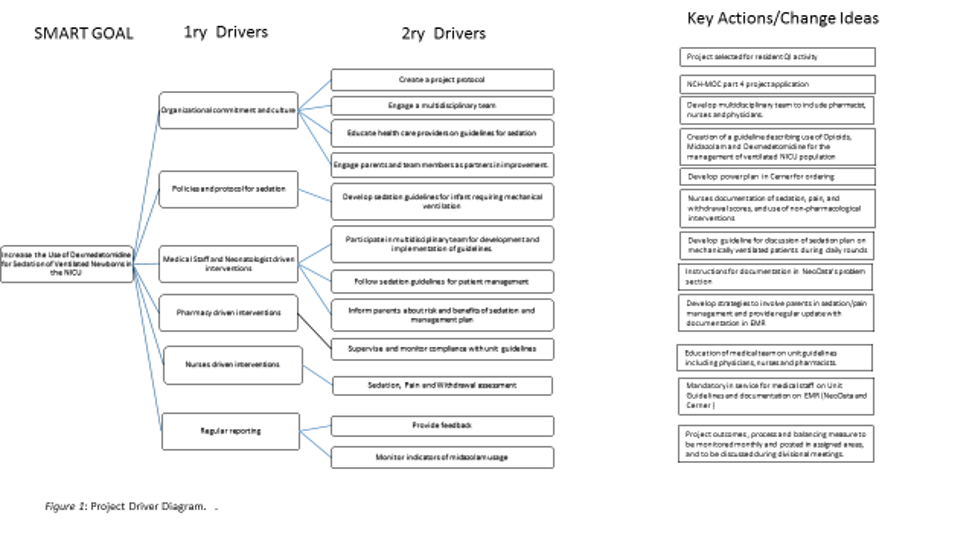
Design/Methods: We adapted the sedation clinical pathway developed by Children’s Hospital of Philadelphia (1). We favored administration of fentanyl and dexmedetomidine over midazolam. For infants with hypoxic ischemic encephalopathy, we recommended dexmedetomidine over opioids. The project driver diagram is shown in figure 1. Pre- and post-intervention data was obtained respectively from January to June 2022 (retrospectively) and from July 2022 to June 2023 (prospectively). The QI targeted newborns requiring sedation for MV >48h at a PCA ≥34 weeks; we excluded PICU transfers, infants on palliative care and those requiring MV longer than 28 days.
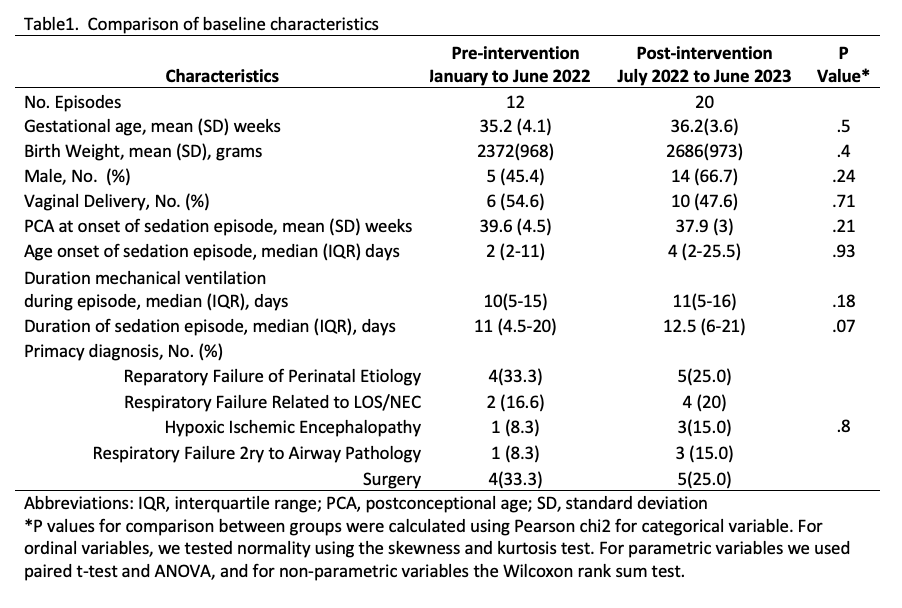
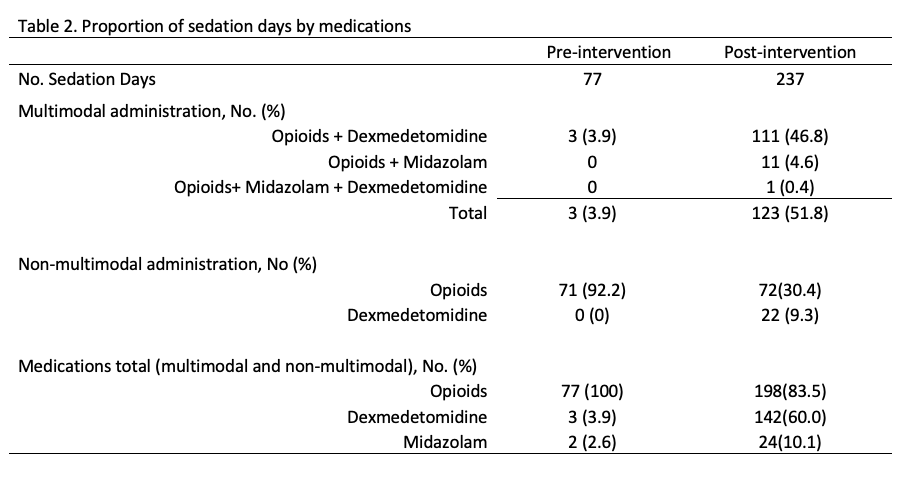
Results: The patient characteristics for both groups are shown in Table 1. The proportion of sedation days infants received multimodal administration of sedation increased from 4% to 52%; favoring the administration of opioids and dexmedetomidine over opioids and midazolam. Non-multimodal administration of sedatives decreased for opioids from 92% to 30% and increased for dexmedetomidine from 0% to 9.3%. The rise on dexmedetomidine is expected given recommendation for use in HIE. Regarding the secondary outcome, we decreased the overall use of sedation days with opioids by 17%. The use of dexmedetomidine increased by 56% whilst midazolam only by 7%.
Conclusion(s): The intervention was successful in increasing the percentage of sedation days with a multimodal approach of sedation using opioids and dexmedetomidine and consequently reducing the exposure to opioids with a minimum increase in midazolam administration.