Cardiology
Session: Cardiology 1
142 - Dysregulated Endo-Myocardial Interaction via Aberrant NRG/ERB Signaling in Endocardial Fibroelastosis
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 142
Publication Number: 142.3273
Publication Number: 142.3273

Daniel Diaz-Gil, MD (he/him/his)
Resident
Boston Children's Hospital/Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background: Endocardial fibroelastosis (EFE) is a distinct form of left ventricular (LV) fibrosis commonly seen in congenital heart defects like hypoplastic left heart syndrome (HLHS) and congenital aortic stenosis. It restricts LV growth and function prenatally and progresses to infiltrate into the underlying myocardium. While endothelial-to-mesenchymal transition (EndMT) originating from the endocardium has been described as pathomechanism, the impact of EFE on the underlying myocardium remains poorly understood.
Objective: The primary objective of this study was to investigate the interactions between the endocardium and myocardium in patients with EFE.
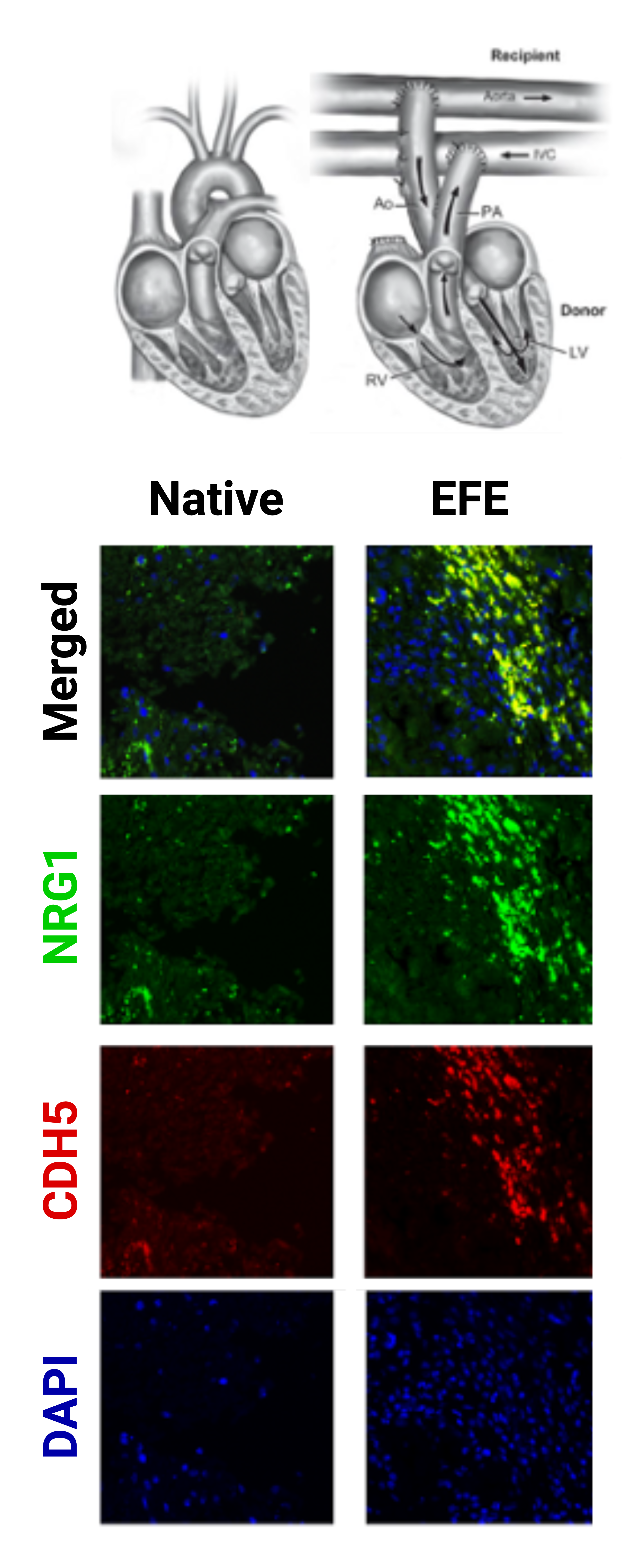
Design/Methods: We conducted single nucleus RNA sequencing (snRNA-seq) on cardiac tissue from 7 participants with EFE and 4 age-matched participants without congenital heart disease. Additionally, we validated our findings via immunohistochemistry in a heterotopic transplantation model designed to simulate the early onset of EFE.
Results: Nine cell types were identified in 77,612 LV nuclei isolated from EFE and control tissues. Fibroblast, endothelial cells, and cardiomyocyte cell clusters were further analyzed. Among these cell types, we identified 24 distinct transcriptional states. EFE-tissue showed significantly increased fibroblasts (p < 0.05), specifically in cell states with transcriptional activation of extracellular matrix remodeling genes and upregulation of neuregulin-1 (NRG1, log2FC 3.89, expression in 45.7% vs 7.4% of EFE vs control nuclei, adjusted p< 0.0001, Fig.1). We also observed alterations in EFE endocardial endothelial cells, including upregulation of neuregulin-3 (NRG3, log2FC 1.52, expression in 91.3% vs 46.2% of EFE vs. control nuclei, adjusted p=0.0219, Fig.1), and differential expression of genes enriched for the ERBB2/4 signaling pathway. The NRG1 receptor ERBB4 was downregulated in EFE cardiomyocytes compared to controls (log2FC range -2.83 to -0.73, adjusted p< 0.05, Fig.1). In our animal model simulating early onset EFE, NRG1 upregulation was detected within the EFE tissue but not in controls (Fig. 2).
Conclusion(s): Our study provides insights into the molecular alterations associated with EFE, particularly the upregulation of NRG1 and its potential role in affecting the underlying myocardium via altered ERB signaling. These findings contribute to a better understanding of the endo-myocardial interplay in EFE, potentially opening new avenues for the development of targeted therapies and interventions.
.png)