Neonatology
Session: Neonatal Infectious Diseases/Immunology 2
613 - The efficacy of antibiotic regimens for the treatment of pneumonia among young infants aged <60 days: a systematic review.
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 613
Publication Number: 613.103
Publication Number: 613.103

Krysten North, MD, MPH
Instructor
Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background: Pneumonia is associated with about 200,000 neonatal deaths annually. Most pneumonia-related deaths among young infants are thought to be preventable through early and optimal treatment. However, an understanding of the optimal treatment regimen is lacking.
Objective: To determine the efficacy of currently recommended antibiotic regimens for pneumonia among young infants compared to alternate regimens.
Design/Methods: We conducted a systematic review of published results from randomized controlled trials (RCTs) that evaluated antibiotic regimens for pneumonia among young infants aged < 60 days in all settings. We searched MEDLINE, Embase, CINAHL, the World Health Organization (WHO) Global Index Medicus, and the Cochrane Central Registry of Trials for published RCTs with no restriction by date or language of publication. Pneumonia was defined according to each trial’s reported definition, including isolated fast breathing. We used standard Cochrane methods and determined the certainty of evidence based on GRADE standards. Due to heterogeneity in trial interventions and outcomes, a meta-analysis was not conducted.
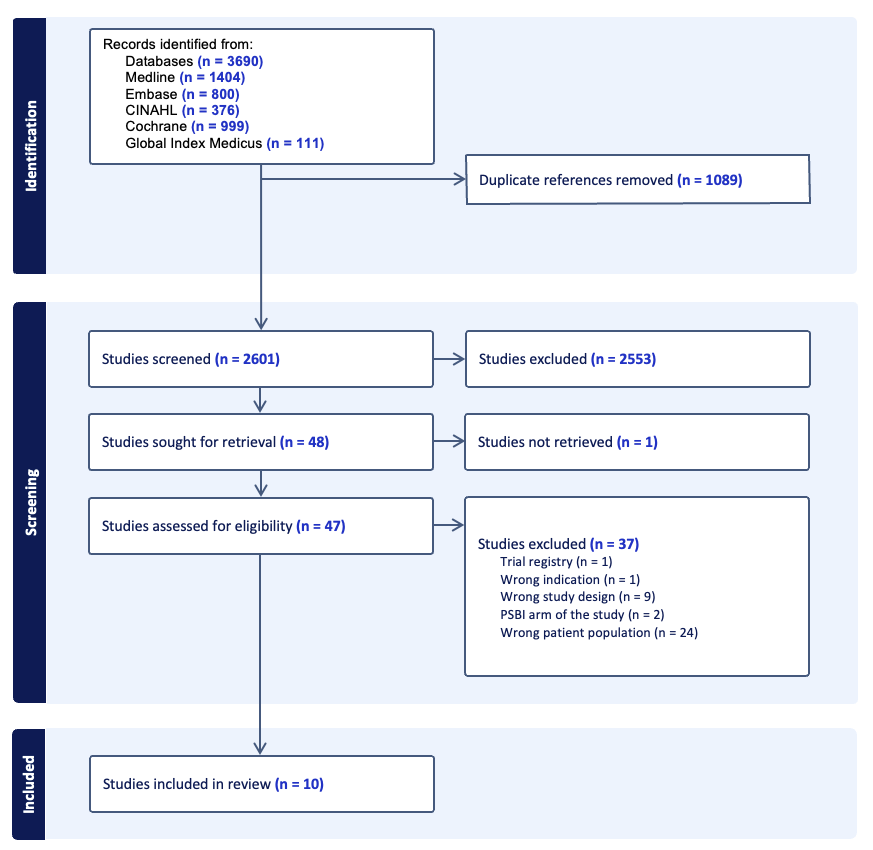
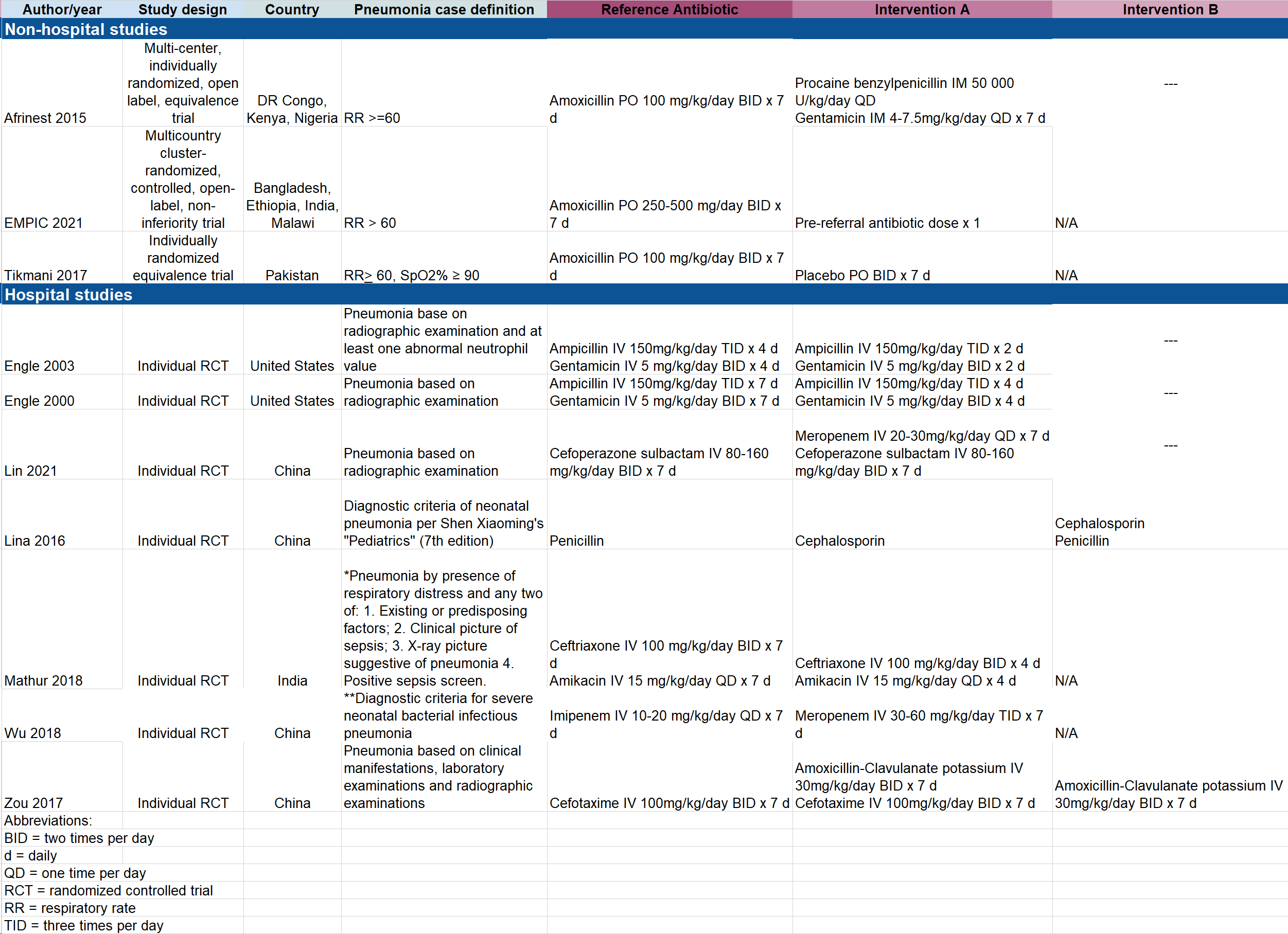
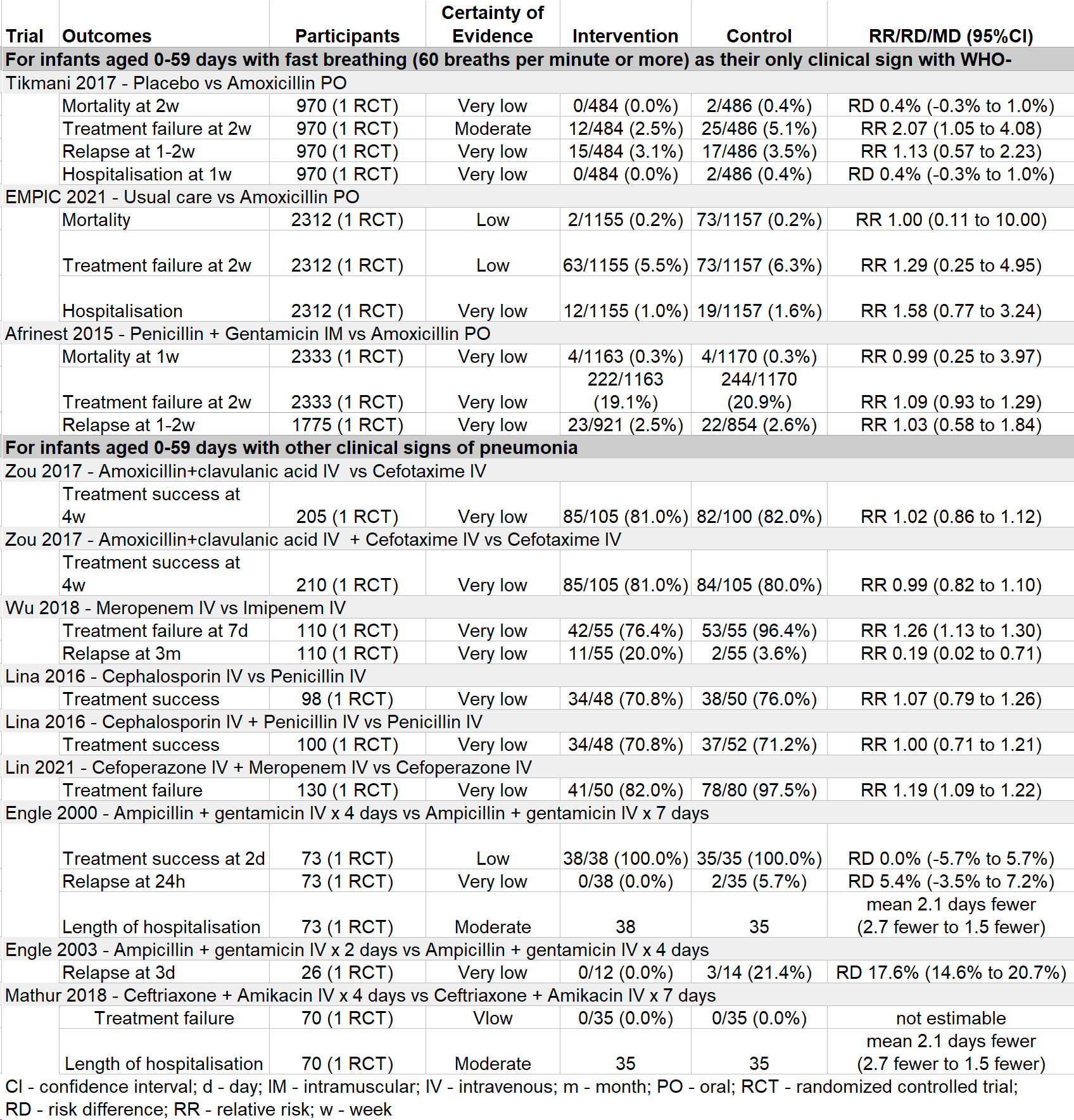
Results: Of 2,601 studies related to antibiotics for pneumonia in young infants, we identified 10 RCTs that met inclusion criteria (Figure 1). Study characteristics are described in Table 1. These trials were conducted in 10 countries in sub-Saharan Africa, Asia, and the United States and evaluated a total of 12 different antibiotic regimens. Seven studies were hospital-based and three studies were non-hospital based. No study evaluated WHO-recommended first choice regimens for hospital treatment of pneumonia. One study evaluated a WHO second-choice regimen (Zou 2017: amoxicillin-clavulanate +/- cefotaxime versus cefotaxime). Three of the hospital-based trials evaluated the duration of antibiotic therapy. Among non-hospital-based trials, one trial compared the efficacy of oral amoxicillin to placebo, one trial compared oral amoxicillin to penicillin and gentamicin, and one study compared oral amoxicillin to standard hospital referral. Individual outcomes, effect sizes, and certainty of evidence are described in Table 2. Overall, certainty of evidence was low or very low for nearly all outcomes.
Conclusion(s): Definitive clinical trials testing the optimal antibiotic regimen for pneumonia among young infants are lacking, including trials to support current recommendations. Additional studies are warranted to determine the most optimal antibiotic regimen to effectively treat pneumonia among young infants.