Infectious Diseases
Session: Infectious Diseases 2
67 - Evaluation of sepsis associated outcomes with mono- vs dual- therapy in pediatric MRSA sepsis
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 67
Publication Number: 67.1496
Publication Number: 67.1496

Maeve Woeltje, MD (she/her/hers)
Fellow
UPMC Childrens Hospital of Pittsburgh
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Background: Sepsis due to Methicillin-resistant Staphylococcus aureus (MRSA) is associated with high morbidity and mortality among children. A prior observational study of hospitalized children co-infected with MRSA and influenza showed a survival benefit with the use of vancomycin plus an additional anti-MRSA antibiotic as part of the initial treatment.
Objective: We examined the prevalence of mono- and dual-anti-MRSA antibiotic regimens in a multicenter dataset of children with sepsis. This is a first step to determine if it is possible to build sufficient cohort sizes with probabilistic linkage for future analyses.
Design/Methods: This retrospective cohort study identified children with sepsis in the Improving Pediatric Sepsis Outcomes (IPSO) Database and incorporated additional data for each encounter by performing probabilistic linkage with the Pediatric Health Information Systems (PHIS) Database. Exclusion criteria were inability to link data, transfer from an outside hospital, age ≤ 60d or ≥19y, and unknown time of sepsis onset. Patients in the cohort with confirmed or possible MRSA, with and without influenza, were identified using ICD-10 codes. Anti-MRSA antibiotic regimens were divided into four groups: mono- vs dual- therapy, with or without vancomycin. Treatment regimens were defined based on antibiotics administered ± 1-day or ± 3-days from the day of documented sepsis onset to evaluate whether broadening the initial treatment window substantially changed the number in each group.
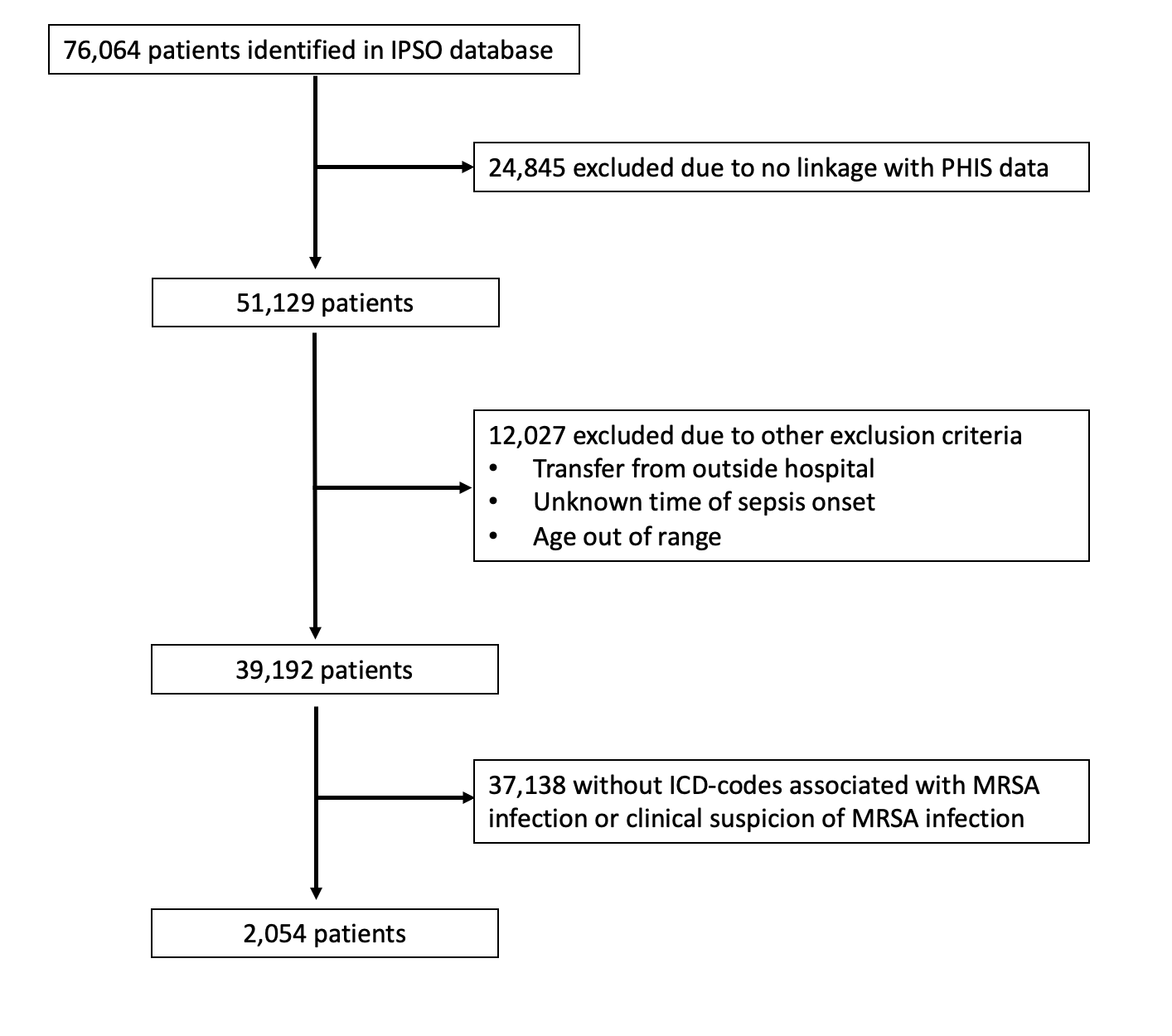
Results: Of 76064 IPSO patients, 2054 from 28 hospitals were included. Most exclusions were due to an inability to link or no MRSA infection (Figure 1). Median age was 8y (IQR 2-13y) and 53.4% were male. Vancomycin monotherapy was the most common regimen in both the ±1-day and ±3-day treatment windows (Tables 1 and 2). Dual therapy including vancomycin was the second most common regimen. Patients with possible MRSA were more likely to receive monotherapy without vancomycin than those with confirmed MRSA. Dual therapy regimens were more common in the ±3-day treatment windows.
Conclusion(s): Among children with sepsis and a MRSA-related ICD-10 code identified across 2 probabilistically linked, multicenter databases, a substantial proportion of patients received dual anti-MRSA therapy as part of an initial antimicrobial regimen around the onset of sepsis. These cohorts appear promising to allow further investigation into whether early dual therapy is associated with better outcomes compared to vancomycin monotherapy. In broadening the initial treatment window to ±3-days, we may be able to include more patients with dual therapy.
.jpg)
.jpg)
