Adolescent Medicine
Session: Adolescent Medicine 3
167 - Awareness, attitudes, and use of HIV Pre-Exposure Prophylaxis in adolescents with recent sexually transmitted infection enrolling in an HIV prevention intervention trial
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 167
Publication Number: 167.241
Publication Number: 167.241

Sarah Wood, MD, MS (she/her/hers)
Assistant Professor
Perelman School of Medicine at the University of Pennsylvania
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Adolescents with sexually transmitted infections (STI) are at disproportionate risk of HIV, yet uptake of HIV pre-exposure prophylaxis (PrEP) remains limited in this population.
Objective: We investigated PrEP awareness, knowledge, willingness and intentions to use PrEP, and prior PrEP use among adolescents with recent STI(s) enrolling in a randomized controlled trial testing a behavioral intervention.
Design/Methods: The TAKE (Treat Act Know Engage) Steps trial enrolled 80 adolescents (ages 13-19) within 30 days of gonorrhea, chlamydia, syphilis, and/or trichomonas treatment at five primary care practices in Philadelphia, PA beginning September, 2022. Participants completed baseline surveys including sociodemographic characteristics and HIV risk behaviors. Prior to asking about PrEP, we provided a brief explanation (i.e., PrEP is highly effective oral or injectable HIV prevention medication). Participants then self-reported their awareness, knowledge, and past use of PrEP. We assessed willingness to use oral and/or injectable PrEP (i.e. would they consider using PrEP), and for participants not willing or unsure, queried why. A validated single item asked intentions to start PrEP in the next 3 months. Exploratory logistic regression assessed associations between intentions to start PrEP and objective HIV risk factors including concern for substance use disorder (CRAFFT total score>3), lifetime number of STIs, and inconsistent condom use.
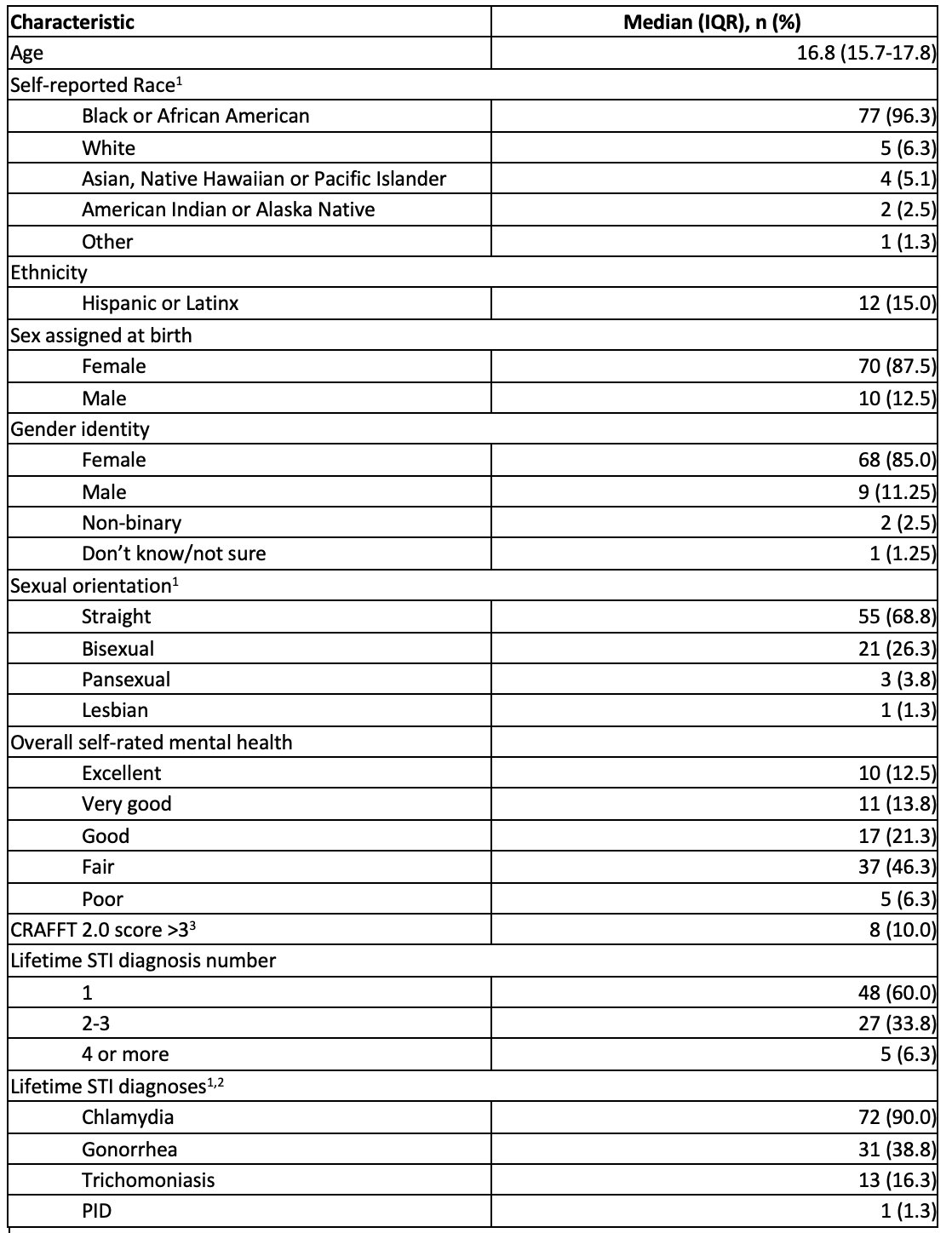
Results: Sociodemographic characteristics of the sample are in Table 1. Only 7% of the sample had heard of, and only n=1 had taken PrEP and had since discontinued. Nearly half (46%) were willing to use oral and 65% willing to use injectable PrEP. Over one-third (35%) agreed with the statement “I intend to start PrEP in the next 3 months.” Among adolescents unwilling to use PrEP, the most common reasons were not perceiving themselves as being at risk of HIV, not wanting to take medication regularly, and concern about side effects. Only 35% correctly answered a question about PrEP effectiveness and only 31% correctly answered that PrEP would not protect against other STIs. In our exploratory analysis (n=80), we found no significant association between HIV risk factors and intention to use PrEP in our sample.
Conclusion(s): Among youth with recent STIs enrolled in an HIV prevention trial, PrEP awareness and use were very low. Encouragingly, most were willing to use PrEP, with higher interest in injectable PrEP. Our findings underscore the need for multi-level interventions to support PrEP uptake for adolescents with STIs.