Sedation Medicine
Session: Sedation Medicine
79 - Gabapentin for Sedation and Pain Control in Neonates after Mandibular Distraction Osteogenesis
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 79
Publication Number: 79.1456
Publication Number: 79.1456

Madison D. Wahl, BA (she/her/hers)
Medical Student
Oregon Health & Science University School of Medicine
PORTLAND, Oregon, United States
Presenting Author(s)
Background: Opioids have been used in the front lines of pain management in neonates, but are not without side effects, notably respiratory depression and decreased gastrointestinal motility. Gabapentin is emerging as a possible alternative medication to potentially decrease opioid exposure, but there remains little data on its use or outcomes in neonates.
Objective: To investigate clinical outcome differences among infants receiving gabapentin instead of opioids as first-line pain management after a mandibular distraction osteogenesis (MDO)
Design/Methods: This was a retrospective cohort study of infants who underwent an MDO between January 1, 2010 to December 31, 2021 in a single center. Chart review was conducted, grouping infants by use of gabapentin. Data was analyzed with SPSS statistical software. Primary outcome was use of post-operative morphine infusion. Secondary outcomes included additional medication exposure, time on respiratory support, time to feeds, pain scores, and medication adverse effects.
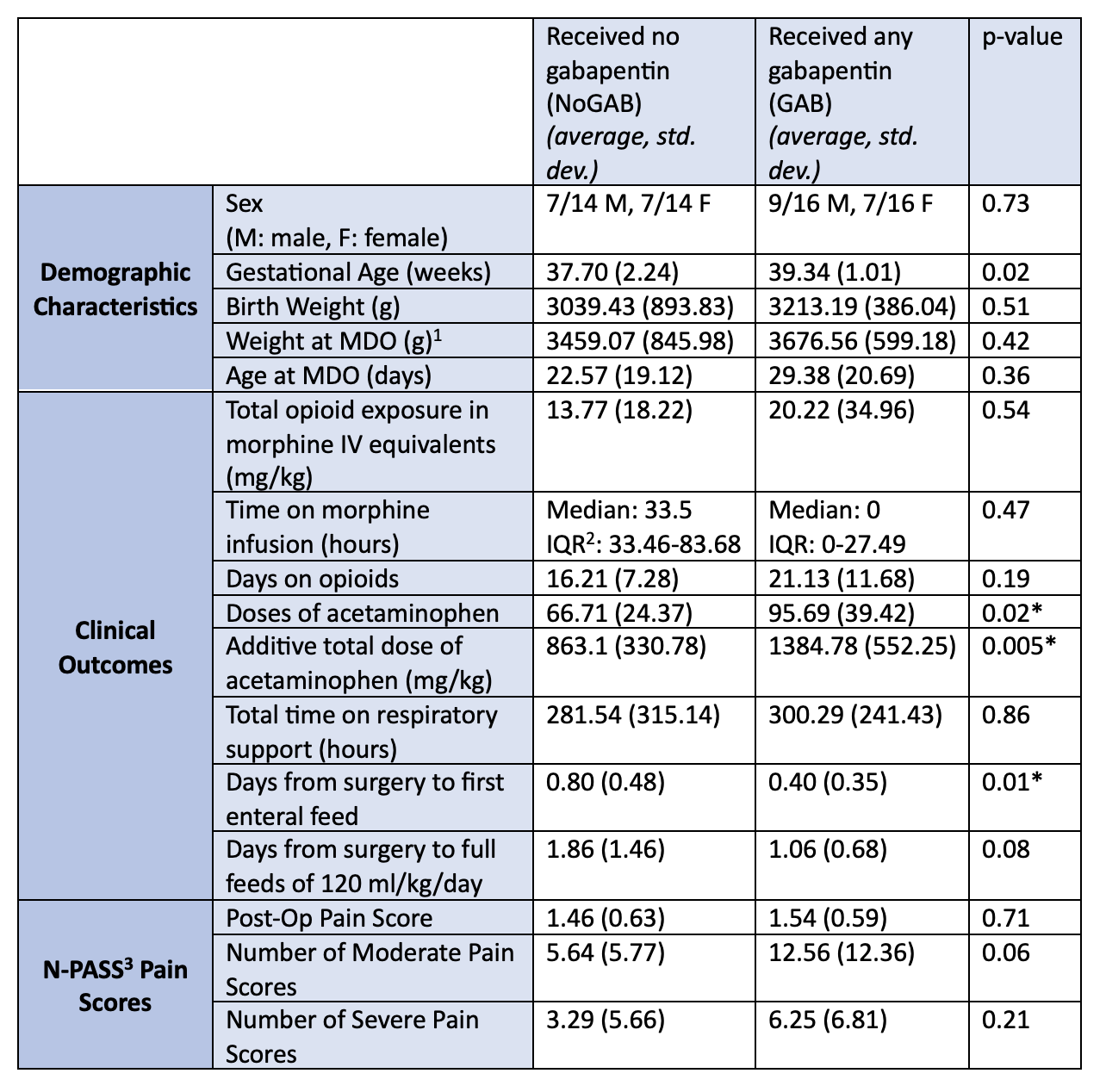
Results: Thirty infants met inclusion criteria. Characteristics were similar between the two groups except for mean gestational age at birth (gabapentin group (GAB), 39.34 +/- 1.01 weeks, vs the no gabapentin (NoGAB) group, 37.70 +/- 2.24 weeks, p= 0.02). We found no difference in use of opioid infusions between GAB (38%) versus NoGAB (71%, p= 0.06), although the median duration of opioid infusion trended lower in the GAB group. GAB infants received more acetaminophen doses (95.69 vs 66.71, p= 0.02), had a greater additive total acetaminophen dose (1384.78 mg/kg vs 863.1 mg/kg, p= 0.005), and had a shorter time to first post-op enteral feed (0.4 days vs 0.8 days, p= 0.01). There were no differences in secondary outcomes (Table 1), or adverse outcomes (Table 2) between the two groups.
Conclusion(s): We saw no difference in total opioid exposure between the two groups despite a trend towards shorter duration of opioid infusions in the GAB group. This could be due to a small sample size lacking power to detect a difference in our primary outcome or a difference in practice with intermittent opioid dosing replacing opioid infusions. The shorter time to first post-op feed in the GAB group suggests that using it may avoid some of the adverse effects of opioids, like delayed gastrointestinal motility. The low number of adverse outcomes in both groups and similar average pain scores is reassuring, but the lack of difference in overall opioid exposure in the GAB group points to the need for further investigation into the efficacy of gabapentin for MDO pain control and re-evaluation of post-surgical practice patterns.
.png)
