Quality Improvement/Patient Safety
Session: Quality Improvement/Patient Safety 3
4 - Ensuring Timely Adoption of Thyroid Screening After Iodinated Intravenous Contrast in Young Children
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 4
Publication Number: 4.3110
Publication Number: 4.3110

Christine Klingaman, DO
Pediatric Hospital Medicine Fellow
Inova Children's Hospital
Fairfax, Virginia, United States
Presenting Author(s)
Background: As of March 30, 2022, the FDA recommends thyroid function testing (TFT) for children < 4 years of age within 3 weeks of receiving IV iodinated contrast due to an increased risk of thyroid dysfunction and potential for future developmental delays. Frameworks ensuring routine lab follow up in a cohort of patients not easily identified by a common diagnosis are not well studied. Multi-faceted solutions are needed for cross-encounter providers to identify at-risk patients, to recall the timing and tests needed, to interpret results, and to communicate the information to primary care providers (PCP) and caregivers.
Objective: To increase compliance with TFT completion or communicating the need for TFTs in patients < 4 years receiving IV iodinated contrast to 20% in 3 months and 75% in 12 months.
Design/Methods: Stakeholders were gathered and primary drivers of success were agreed upon. The process was mapped out across encounters to identify opportunities and barriers. Measures included the percentage of at-risk patients for whom there was communication regarding the need for TFTs with PCPs via the discharge (DC) summary, caregivers via the after visit summary (AVS), and TFTs completed within the recommended timeframe. EMR tools including conditional, automated SmartText in radiology results, inpatient AVS and DC summaries were used to identify at-risk patients and improve cross-encounter communication. The Model for Improvement was utilized testing changes through PDSA cycles.
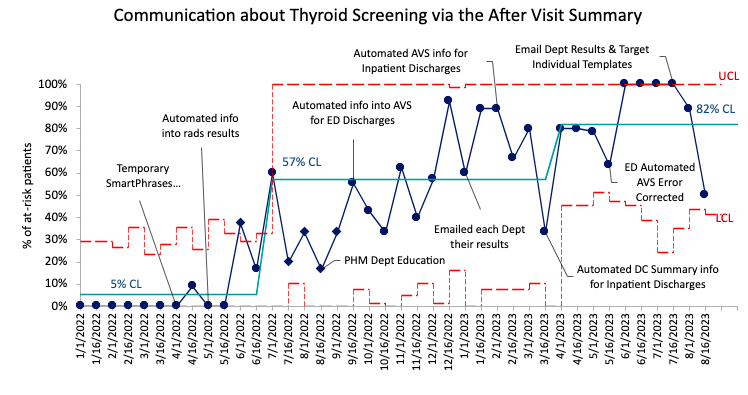
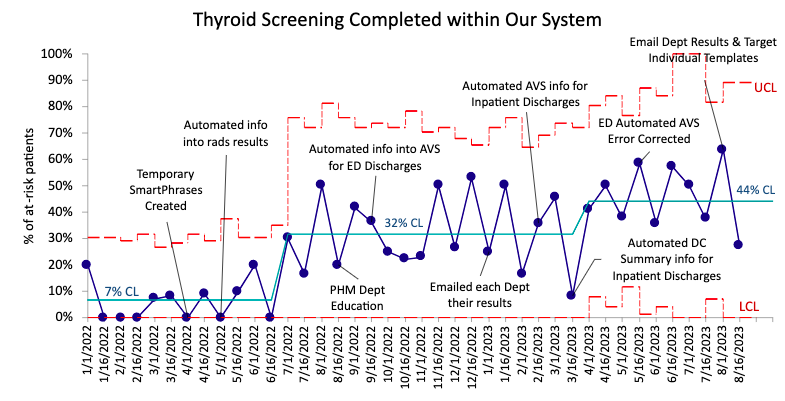
Results: We identified 471 children who met inclusion criteria. Of these, 43% had high-risk comorbidities (age < 3 months, VLBW or congenital heart disease). Compliance with communicating the need for TFTs with PCPs through DC summaries increased from a mean of 4% initially to 64% and with caregivers through the AVS from a mean of 5% to 82% in 20 months. The percentage of patients with TFTs completed within our system in the appropriate interval increased from a mean of 7% to 44%, of which 23% were abnormal or underwent further testing. To date, two patients have required treatment that were not previously treated for hypothyroidism.
Conclusion(s): This project has been successful in achieving its aim. Data collection is limited by the fact that only lab results within our system could be reviewed for completion. We expect further improvement following upcoming BPA implementation which will aid providers in identifying at-risk patients and ordering testing at the appropriate interval.
.png)